Abstract
Field emission scanning electron microscopy (FE-SEM) and X-ray diffraction (XRD) have been used to study the microstructural changes and phase development that take place during the hydration of cubic (pure) and orthorhombic (Na-doped) tricalcium aluminate (C3A) and gypsum in the absence and presence of lime. The results demonstrate that important differences occur in the hydration of each C3A polymorph and gypsum when no lime is added; orthorhombic C3A reacts faster with gypsum than the cubic phase, forming longer ettringite needles; however, the presence of lime slows down the formation of ettringite in the orthorhombic sample. Additional rheometric tests showed the possible effects on the setting time in these cementitious mixes.
Similar content being viewed by others
Introduction
Cement chemistry uses the term “hydration” to denote the changes that occur when the anhydrous cement, or one of its phases (the so-called clinker phases), are mixed with water. This involves a complex group of reactions that cause changes in the chemical and physico-mechanical properties of the system, particularly in the setting and hardening of the mix [1]. The hydration process is sensitive to many factors, among which clinker composition is of special relevance because it controls the kinetics of cement hydration [2]. The knowledge of the hydration behavior of a pure phase and its interaction with other phases form the basis of the interpretation of the complex reactions, which occur in the Portland cement hydration under various conditions.
The reaction of tricalcium aluminate (C3A) with water is instantaneous. Crystalline hydrates, such as C3AH6, C4AH19, and C2AH8, form quickly, generating large amounts of heat [3 and references therein]. This happens even in the presence of retarders, as observed by means of 27Al MAS NMR [4]. To slow down the hydration reaction, calcium sulfate is added to the mix, which causes ettringite to form. Ettringite is a calcium sulpho-aluminate \( \left( {{\text{C}}_{ 6} {\text{A}}\overline{\text{S}}_{6} {\text{H}}_{32} } \right) \) that forms during the first stages of hydration and subsequently develops a three-dimensional nanocrystal network, which ultimately leads to setting and hardening of the cement paste. If hydration of C3A in cement is not controlled, setting occurs very rapidly and the resulting mix cannot be used for most construction applications because of its high viscosity and low workability.
Industrial clinkers contain minor elements such as Na2O, K2O, SiO2, Fe2O3, SO3, and Cl−, coming from raw materials, fuel, and the lining of the kilns [5, 6]. These alkalis can be incorporated into a number of phases in the clinker, and often Na2O is normally taken up by the C3A [7]. When C3A is synthesized in the presence of these elements, it causes changes in its crystal lattice, and the formation of the other phases occurs. There are several solid solutions of general formula Na2xCa3−xAl2O6, such as cubic, monoclinic, orthorhombic, and tetragonal [8–10], but in cements, the cubic or orthorhombic C3A are found alone or in combination [11].
Numerous studies have been conducted to quantify the influence of these polymorphs (cubic and orthorhombic) on the mechanisms of cement hydration, such as those by Boikova and Domansky [10]; Regourd et al. [12]; Odler and Wonnemann [13]; Massazza and Daimon [6]; Samet and Sarkar [14]; and Stephan and Wistuba [15]. Despite these studies, however, a consensus has not been reached, and a sizeable knowledge gap still exists concerning the specific characteristics of each polymorph, having a marked effect on their reactivity.
Although C3A is a small component of most clinkers (commonly 4–10 wt%), the content of this aluminate can reach values exceeding 10 wt% in white Portland cement (WPC) [16]. The high concentration of C3A in the composition of WPC affects the setting time of this cement, resulting in a normally shorter setting time compared to ordinary Portland cement (OPC) [17]. Moreover, these high levels must also be taken into account regarding long-term durability issues, as the reactivity of calcium aluminate with sulfate-bearing solutions make high-C3A cements especially prone to develop a series of deleterious reactions known generically as “sulfate attack”. Therefore, detailed microstructural and mineralogical studies of the hydration behavior of C3A polymorphs are of major importance in understanding the complexity of the setting mechanisms of cements with relatively high contents of tricalcium aluminate. To better understand how the presence of gypsum and lime affect the hydration of cubic and orthorhombic C3A, this study conducted a detailed FE-SEM, XRD, and rheological study of the hydration behavior of synthetic cubic and orthorhombic C3A in the presence of gypsum, with and without the addition of lime.
Experimental section
Materials
Samples of tricalcium aluminate (C3A), orthorhombic and cubic, were obtained from Construction Technology Laboratories, Inc., located in Skokie, Illinois. The pure compounds of orthorhombic and cubic C3A were synthesized in the laboratory by heating a stoichiometric blend of reagent grade CaCO3 and alumina (Al2O3) in an electric furnace at 1,400 °C for 1 h, followed by quenching in air. The orthorhombic C3A is composed of Na2Ca8Al6O18; to synthesize it, the reagent grade Na2CO3 was also used as a raw material. After synthesizing, each material was ground in a ceramic mill to 325 meshes. The powders were characterized by means of thermo-gravimetric analysis, X-ray fluorescence, XRD, and laser granulometry methods. The gypsum (CaSO4 · 2H2O) and lime (CaO) used in this study were provided by Fisher Scientist, located in Pittsburgh, Pennsylvania.
Methods
For the FE-SEM and XRD analysis, the samples were hand mixed for 2 min and then stored at 100% RH, and covered with a plastic film until they were studied 7 or 14 days later. The amount of each compound (gypsum and both C3A) was stoichiometrically calculated based on the quantity necessary to form ettringite, which resulted in a 1:1.9 ratio (C3A:gypsum); the water content was 0.6 (water-to-solid materials ratio—w/s). Ten percent lime with respect to C3A amount was added in the pastes to observe how increasing the system alkalinity affects ettringite formation. Table 1 shows how the mixes proportions were set in the experiments.
Selected samples of these pastes were sputtered with gold and investigated with a Hitachi S-5000 FE-SEM.
X-ray investigations were carried out with a Phillips XPert MPD PW3373/00 diffractometer operated at an accelerating voltage of 40 keV on a CuKα anode, irradiation intensity of 40 mA, and 40o scans in steps of 0.02o/s.
The evolution of early hydration of additional mixes prepared with the same material ratios was studied with a Haake RheoStress 600 rheometer (RT) with plate–plate configuration, operating in controlled strain mode. The rotating plate was 8 mm in diameter, and the side in contact with the sample was grooved in order to prevent possible slippage. The samples were hand mixed for 2 min before placing them on the fixed lower plate. The upper plate was then lowered down to a final thickness of the sample of 2 mm. The excess of sample was removed from the lower plate. Next, a shear rate of 600 s−1 was applied for 30 s; then during the next 60 s the samples remained still; and, finally, a sweep of strains was applied at a fixed frequency of 5 rad/s. The total time elapsed for each test was 14 min and started immediately when the hydration began. The temperature on the fixed plate was kept at 20 ± 1 °C. This configuration was based on Nachbaur et al. [18], Schmidt and Schlegel [19], and Sun et al. [20].
Results
Microscopic examination and XRD measurements
The morphological study of ettringite formation in the systems of cubic and orthorhombic C3A, gypsum, and lime was performed with a FE-SEM and XRD, each system was presented separated to better understand the results.
Hydration of cubic C3A and gypsum
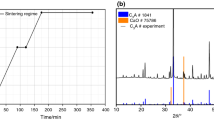
Figure 1 presents FE-SEM micrographs of a paste consisting of cubic C3A and gypsum, hydrated for 7 and 14 days, with a w/s = 0.6. The pictures show that at 14 days, the sample still contains tricalcium aluminate and gypsum, also confirmed by XRD analysis (Fig. 2b). Ettringite, forming stubby crystals of up to a few hundreds of nanometers in length, is clearly evident in association with C3A grains. After 14 days, the crystals are longer (typically ≥1 μm) and more abundant. Phase confirmation of ettringite is obtained from its low-angle X-ray tag (d-spacing = 9.8 Å) that allows it to be easily distinguished from residual gypsum (7.7 Å). This paste after 7 days remains wet in the sample holder.
In a similar study, Bishop et al. [4] found that the ettringite rods generally range from 1 to 2 μm in length and are about 10–20 nm wide when grown in situ from pastes of cubic C3A and gypsum.
Hydration of cubic C3A + gypsum + 10% lime
Figure 3 shows the FE-SEM micrographs of a paste with cubic C3A, lime, and gypsum hydrated for 7 and 14 days. After 14 days, gypsum and C3A are still present, also confirmed by XRD analysis (Fig. 4b). The sample presents the same behavior as the mix without lime. In this micrograph, the ettringite is evident close to the particles of C3A and gypsum, and formation of portlandite (CH) is also clear. The crystal size of ettringite is, again, of a few hundreds of nm in length.
Hydration of orthorhombic C3A + gypsum
Figure 5 shows the appearance of the pastes after 7 days. The paste with orthorhombic C3A is dry and shows little expansion when compared to the paste with cubic C3A in the same mix proportion. Figure 6 presents the FE-SEM micrographs of pastes of orthorhombic C3A and gypsum hydrated for 7 and 14 days. The images indicate that at 7 days, the samples apparently contain no residual gypsum, but peaks of small intensity of gypsum are detected in XRD (Fig. 7a). These FE-SEM images demonstrate that orthorhombic C3A reacts at a faster rate when compared with the cubic C3A pastes in the same proportion, because all the gypsum was consumed by 7 days of hydration. The ettringite formation can be seen by the dissolution of C3A and consumption of all gypsum on the sample after 14 days (Fig. 7b). When compared to pastes with cubic C3A, in presence and absence of lime, the crystal size is longer, ranging between 1 and 4 μm in length at both ages, 7 and 14 days.
Hydration of orthorhombic C3A + gypsum + 10% lime
Figure 8 presents the FE-SEM micrographs of a paste of orthorhombic C3A, lime, and gypsum hydrated for 7 and 14 days. The XRD patterns show that at 14 days (Fig. 9b), the sample still contains gypsum and C3A. The behavior of orthorhombic C3A + gypsum in presence of 10% of lime resulted in a retardation of ettringite formation. The formation of CH occurred, and the crystals size of ettringite was smaller (1 μm in length) when compared to the paste of orthorhombic C3A with no lime.
Oscillatory rheometric measurement
Figure 10 shows the evolution of the storage modulus (G′) of freshly prepared mixes of the two polymorphs of C3A hydrating in the presence of gypsum. The stiffness was higher in the orthorhombic C3A mix from the beginning of the test and, most importantly, increased at a higher rate than that of the cubic C3A-based mix during the very first minutes of the hydration. Thus, an average increase of 158 Pa/s was measured from the former, whereas 97 Pa/s was obtained from the latter. These results may well suggest that a faster hydration of the orthorhombic C3A is evidenced from the very early stage of the process and, as a consequence, the mix tends to set earlier in this case. Likewise, this is in accordance with the results from FE-SEM and XRD obtained at much later ages.
Discussion
The images and the XRD patterns demonstrate that the pastes containing orthorhombic C3A reacted at a faster rate. By the seventh day of hydration, all the gypsum had been consumed, and by 14 days, the XRD diffraction showed only peaks of ettringite on the orthorhombic C3A paste, whereas after 14 days, traces of gypsum and cubic C3A remain in the paste with this aluminate.
Interestingly, the ettringite formation appeared to be retarded in both C3A pastes that contained lime, being much more retarded in the orthorhombic sample when compared with the correlate sample with no lime. Also the size of ettringite crystals was smaller when compared with those obtained from mixes without lime.
These observations may be explained in terms of the pH. Goetz-Neunhoeffer et al. [2] stated that depending on pH, two extreme crystal morphologies of ettringite could be determined. When the pH is 9.5 in solution, the precipitated ettringite crystals exhibit a very thin extremely acicular shape and are grown together in the direction of c-axis. The hexagonal prisms were up to 110 Å in length. Changing the pH to 12.5, by addition of sodium hydroxide, led to more prismatic hexagonal crystals, which achieved lengths up to 20 Å in direction of c-axis.
In this research, the addition of lime resulted in the formation of Ca(OH)2, which led to an increase in the pH. The presence of Ca(OH)2 changes considerably the course of hydration of C3A, as this compound reduces the solubility of Al, thus increasing the pH [6, 21] .
Based on these results, when the alkaline level is increased because of an increase in the amount of alkalis in the paste—in this study by adding lime to the mixture—the normal dissolution rate of C3A does not occur and, consequently, inhibits the formation of ettringite.
Another factor to consider is the difference between the crystalline structures of cubic and orthorhombic sample. Orthorhombic C3A lends itself to selective removal of Na from its structure, creating two zones of attack; a surface layer or zone that is enriched in Al, relative to the bulk composition and, at greater depth, a zone whereby Ca and Al remain, but from which Na is actively removed by selective hydrolysis [22]. This selective removal explains the shorter reaction time of the orthorhombic C3A; the relatively easy dissolution of the protective layer of the grain leads to a faster formation of ettringite.
High alkali contents in the clinker phase increase the pH of cement paste, which, because of the common ion effect, decrease the calcium concentration in solution and also promote the dissolution of C3A [23]. Based on the results of this study, the high alkali (Na-doped C3A) content accelerates hydration and the set of the mix. Most of the literature acknowledges that alkalis accelerate early hydration and also increase early strength, which explains the faster hydration of orthorhombic C3A at early ages noted on the rheometric measurements. Conversely, it is generally accepted that alkalis decrease strength at 28 days and later [23–25]. Further research is necessary to explain this apparent contradiction.
Conclusions
An analysis of the hydration of cubic and orthorhombic C3A by FE-SEM and X-ray diffraction (XRD) demonstrated:
-
based on the size and consumption of C3A and gypsum on these pastes the orthorhombic C3A + gypsum presented a faster rate of formation of ettringite crystals, which were also longer;
-
based on the influence of lime on these aluminates hydration, in the orthorhombic C3A paste, the ettringite formation seems to be more retarded. Likewise, the size of ettringite crystals seems to be smaller when compared with the correlated orthorhombic C3A paste with no lime;
-
no monosulfoaluminate formation was observed.
Rheometric measurements showed that orthorhombic C3A mixes presented higher increase in stiffness than cubic C3A at very early stages of hydration. Thus, this crystalline form of Na-doped C3A (orthorhombic), when present in high amounts in the clinker, may cause problems with setting time and workability.
References
Mathur PC (2007) Study of cementitios materials using transmission electron microscopy. PhD Thesis, Faculté des sciences et techniques de l’ingénieur, École Polytechique Fédérale de Lausanne, Lausanne, p 237
Goetz-Neunhoeffer F, Neubauer J et al (2006) Cem Concr Res 36(1):65
Mehta PK, Monteiro PJM (2006) Concrete—microstructure, properties and materials. McGraw-Hill, New York
Bishop M, Bott S, Barron A (2003) Chem Mater 15:3074
Wachtler H-J, Uschold TV et al (1986) Silikattechnick 4:127
Massazza F, Dalmon M (1992) In: 9th international congress chemical cement, New Delhi, India, p 383
Spierings GACM, Stein HN (1976) Cem Concr Res 6(2):265–272
Maki I (1973) Cem Concr Res 3(3):295–313
Regourd M, Gunier A (1975) Revue des Matériaux de Constr 695:201–215
Boikova AI, Domansky AI (1977) Cem Concr Res 7(5):483–492
Taylor HFW (1990) Cement chemistry. Academic Press, London
Regourd M, Hornain H et al (1980) In: 7th international congress chemical cement, Paris, p 477
Odler I, Wonnemann R (1983) Cem Concr Res 13(6):771
Samet B, Sarkar SL (1997) Cem Concr Res 27(3):369
Stephan D, Wistuba S (2006) Cem Concr Res 36(11):2011
Neville AM (1997) Properties of concrete. Pearson Education Limited, Edinburgh Gate, Harlow
Hamad BS (1995) Adv Cem Bas Mater 2(4):161
Nachbaur L, Mutin JC et al (2001) Cem Concr Res 31(2):183
Schmidt G, Schlegel E (2002) Cem Concr Res 32(4):593
Sun Z, Voigt T et al (2006) Cem Concr Res 36(2):278
Ghorab HY, Abou El Fetouh SH et al (1988) Zem Kalk Gips 12:624
Glasser FP, Marinho MB (1984) Proc Br Ceram Soc 35:221
Juenger MCG, Jennings HM (2001) ACI Mater J 98(3):251
Gebhardt R (1995) Cem Concr Aggreg 17(2):145
Burrows R (1999) The visible and invisible cracking of concrete. ACI monograph No. 11. American Concrete Institute, Farmington Hills, MI
Acknowledgements
The authors acknowledge the financial support of CAPES (Fundação Coordenação de Aperfeiçoamento de Pessoal de Nível Superior—Ministério da Educação—Brasil) and CNPq (Conselho Nacional de Desenvolvimento Científico e Tecnológico—Ministério da Ciência e Técnologia—Brasil). Also we are grateful to the staff of Electron Microscope Laboratory at University of California, Berkeley for helping in the acquisition of the images, and Oscar Reyes, from Technical University of Catalonia (UPC) on the rheological measurements. The research was supported by the National Science Foundation (grant CMS-981275) and KAUST.
Open Access
This article is distributed under the terms of the Creative Commons Attribution Noncommercial License which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This is an open access article distributed under the terms of the Creative Commons Attribution Noncommercial License (https://creativecommons.org/licenses/by-nc/2.0), which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
About this article
Cite this article
Kirchheim, A.P., Fernàndez-Altable, V., Monteiro, P.J.M. et al. Analysis of cubic and orthorhombic C3A hydration in presence of gypsum and lime. J Mater Sci 44, 2038–2045 (2009). https://doi.org/10.1007/s10853-009-3292-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10853-009-3292-3