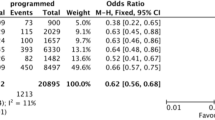
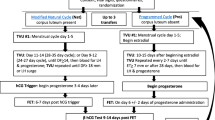
Abstract
Purpose
In the first of two companion papers, we comprehensively reviewed the recent evidence in the primary literature, which addressed the increased prevalence of hypertensive disorders of pregnancy, late-onset or term preeclampsia, fetal overgrowth, postterm birth, and placenta accreta in women conceiving by in vitro fertilization. The preponderance of evidence implicated frozen embryo transfer cycles and, specifically, those employing programmed endometrial preparations, in the higher risk for these adverse maternal and neonatal pregnancy outcomes. Based upon this critical appraisal of the primary literature, we formulate potential etiologies and suggest strategies for prevention in the second article.
Methods
Comprehensive review of primary literature.
Results
Presupposing significant overlap of these apparently diverse pathological pregnancy outcomes within subjects who conceive by programmed autologous FET cycles, shared etiologies may be at play. One plausible but clearly provocative explanation is that aberrant decidualization arising from suboptimal endometrial preparation causes greater than normal trophoblast invasion and myometrial spiral artery remodeling. Thus, overly robust placentation produces larger placentas and fetuses that, in turn, lead to overcrowding of villi within the confines of the uterine cavity which encroach upon intervillous spaces precipitating placental ischemia, oxidative and syncytiotrophoblast stress, and, ultimately, late-onset or term preeclampsia. The absence of circulating corpus luteal factors like relaxin in most programmed cycles might further compromise decidualization and exacerbate the maternal endothelial response to deleterious circulating placental products like soluble fms-like tyrosine kinase-1 that mediate disease manifestations. An alternative, but not mutually exclusive, determinant might be a thinner endometrium frequently associated with programmed endometrial preparations, which could conspire with dysregulated decidualization to elicit greater than normal trophoblast invasion and myometrial spiral artery remodeling. In extreme cases, placenta accreta could conceivably arise. Though lower uterine artery resistance and pulsatility indices observed during early pregnancy in programmed embryo transfer cycles are consistent with this initiating event, quantitative analyses of trophoblast invasion and myometrial spiral artery remodeling required to validate the hypothesis have not yet been conducted.
Conclusions
Endometrial preparation that is not optimal, absent circulating corpus luteal factors, or a combination thereof are attractive etiologies; however, the requisite investigations to prove them have yet to be undertaken. Presuming that in ongoing RCTs, some or all adverse pregnancy outcomes associated with programmed autologous FET are circumvented or mitigated by employing natural or stimulated cycles instead, then for women who can conceive using these regimens, they would be preferable. For the 15% or so of women who require programmed FET, additional research as suggested in this review is needed to elucidate the responsible mechanisms and develop preventative strategies.



Similar content being viewed by others
Data availability
Not applicable.
Notes
Because the use of true or modified NC was not consistently specified in the literature, we combined the two approaches for discussion. See the first article in this two-article series for further details about endometrial preparation in FET.
References
Mumusoglu S, Polat M, Ozbek IY, Bozdag G, Papanikolaou EG, Esteves SC, et al. Preparation of the endometrium for frozen embryo transfer: a systematic review. Front Endocrinol (Lausanne). 2021;12:688237.
Conrad KP, Rabaglino MB, Post Uiterweer ED. Emerging role for dysregulated decidualization in the genesis of preeclampsia. Placenta. 2017;60:119–29.
Garrido-Gomez T, Castillo-Marco N, Cordero T, Simon C. Decidualization resistance in the origin of preeclampsia. Am J Obstet Gynecol. 2022;226(2S):S886–94.
Rabaglino MB, Conrad KP. Evidence for shared molecular pathways of dysregulated decidualization in preeclampsia and endometrial disorders revealed by microarray data integration. FASEB J. 2019;33(11):11682–95.
Brosens I, Pijnenborg R, Vercruysse L, Romero R. The, “great obstetrical syndromes” are associated with disorders of deep placentation. Am J Obstet Gynecol. 2011;204(3):193–201.
Conrad KP. Evidence for corpus luteal and endometrial origins of adverse pregnancy outcomes in women conceiving with or without assisted reproduction. Obstet Gynecol Clin North Am. 2020;47(1):163–81.
Dunk C, Kwan M, Hazan A, Walker S, Wright JK, Harris LK, et al. Failure of decidualization and maternal immune tolerance underlies uterovascular resistance in intra uterine growth restriction. Front Endocrinol (Lausanne). 2019;10:160.
Verlohren S, Melchiorre K, Khalil A, Thilaganathan B. Uterine artery Doppler, birth weight and timing of onset of pre-eclampsia: providing insights into the dual etiology of late-onset pre-eclampsia. Ultrasound Obstet Gynecol. 2014;44(3):293–8.
Khalil A, Garcia-Mandujano R, Maiz N, Elkhouli M, Nicolaides KH. Longitudinal changes in maternal hemodynamics in a population at risk for pre-eclampsia. Ultrasound Obstet Gynecol. 2014;44(2):197–204.
Sohlberg S, Mulic-Lutvica A, Lindgren P, Ortiz-Nieto F, Wikstrom AK, Wikstrom J. Placental perfusion in normal pregnancy and early and late preeclampsia: a magnetic resonance imaging study. Placenta. 2014;35(3):202–6.
Conrad KP, Graham GM, Chi YY, Zhai X, Li M, Williams RS, et al. Potential influence of the corpus luteum on circulating reproductive and volume regulatory hormones, angiogenic and immunoregulatory factors in pregnant women. Am J Physiol Endocrinol Metab. 2019;317(4):E677–85.
Wu H, Zhou P, Lin X, Wang S, Zhang S. Endometrial preparation for frozen-thawed embryo transfer cycles: a systematic review and network meta-analysis. J Assist Reprod Genet. 2021;38(8):1913–26.
Glujovsky D, Pesce R, Sueldo C, Quinteiro Retamar AM, Hart RJ, Ciapponi A. Endometrial preparation for women undergoing embryo transfer with frozen embryos or embryos derived from donor oocytes. Cochrane Database Syst Rev. 2020;10(10):CD006359.
Altmae S, Tamm-Rosenstein K, Esteban FJ, Simm J, Kolberg L, Peterson H, et al. Endometrial transcriptome analysis indicates superiority of natural over artificial cycles in recurrent implantation failure patients undergoing frozen embryo transfer. Reprod Biomed Online. 2016;32(6):597–613.
Young SL, Savaris RF, Lessey BA, Sharkey AM, Balthazar U, Zaino RJ, et al. Effect of randomized serum progesterone concentration on secretory endometrial histologic development and gene expression. Hum Reprod. 2017;32(9):1903–14.
Inversetti A, Mandia L, Candiani M, Cetin I, Larcher A, Savasi V, et al. Uterine artery Doppler pulsatility index at 11–38 weeks in ICSI pregnancies with egg donation. J Perinat Med. 2018;46(1):21–7.
Cavoretto PI, Farina A, Miglio R, Zamagni G, Girardelli S, Vanni VS, et al. Prospective longitudinal cohort study of uterine arteries Doppler in singleton pregnancies obtained by IVF/ICSI with oocyte donation or natural conception. Hum Reprod. 2020;35(11):2428–38.
Choux C, Ginod P, Barberet J, Rousseau T, Bruno C, Sagot P, et al. Placental volume and other first-trimester outcomes: are there differences between fresh embryo transfer, frozen-thawed embryo transfer and natural conception? Reprod Biomed Online. 2019;38(4):538–48.
Cavoretto PI, Farina A, Gaeta G, Sigismondi C, Spinillo S, Casiero D, et al. Uterine artery Doppler in singleton pregnancies conceived after in-vitro fertilization or intracytoplasmic sperm injection with fresh vs frozen blastocyst transfer: longitudinal cohort study. Ultrasound Obstet Gynecol. 2020;56(4):603–10.
Wiegel RE, Karsten MJH, Reijnders IF, van Rossem L, Willemsen SP, Mulders A, et al. Corpus luteum number and the maternal renin-angiotensin-aldosterone system as determinants of utero-placental (vascular) development: the Rotterdam periconceptional cohort. Reprod Biol Endocrinol. 2021;19(1):164.
Conrad KP, Schaub AM, Li M, Qiu Y, Chi Y-Y, Lingis M, et al. Uterine artery Doppler indices and fetal dimensions in women who conceived spontaneously or by in vitro fertilization. Manuscript in preparation.
Burton GJ, Woods AW, Jauniaux E, Kingdom JC. Rheological and physiological consequences of conversion of the maternal spiral arteries for uteroplacental blood flow during human pregnancy. Placenta. 2009;30(6):473–82.
Vieira MC, McCowan LME, Gillett A, Poston L, Fyfe E, Dekker GA, et al. Clinical, ultrasound and molecular biomarkers for early prediction of large for gestational age infants in nulliparous women: an international prospective cohort study. PLoS ONE. 2017;12(6):e0178484.
Plasencia W, Gonzalez Davila E, Tetilla V, Padron Perez E, Garcia Hernandez JA, Gonzalez Gonzalez NL. First-trimester screening for large-for-gestational-age infants. Ultrasound Obstet Gynecol. 2012;39(4):389–95.
Gasiorowska A, Zawiejska A, Dydowicz P, Wender-Ozegowska E, Poprawski G, Tobola-Wrobel K, et al. Maternal factors, ultrasound and placental function parameters in early pregnancy as predictors of birth weight in low-risk populations and among patients with pre-gestational diabetes. Ginekol Pol. 2019;90(7):388–95.
Zhou J, Xiong Y, Ren Y, Zhang Y, Li X, Yan Y. Three-dimensional power Doppler ultrasonography indicates that increased placental blood perfusion during the third trimester is associated with the risk of macrosomia at birth. J Clin Ultrasound. 2021;49(1):12–9.
Olofsson P, Saldeen P, Marsal K. Fetal and uteroplacental circulatory changes in pregnancies proceeding beyond 43 weeks. Early Hum Dev. 1996;46(1–2):1–13.
Farmakides G, Schulman H, Ducey J, Guzman E, Saladana L, Penny B, et al. Uterine and umbilical artery Doppler velocimetry in postterm pregnancy. J Reprod Med. 1988;33(3):259–61.
Xiong X, Demianczuk NN, Buekens P, Saunders LD. Association of preeclampsia with high birth weight for age. Am J Obstet Gynecol. 2000;183(1):148–55.
Xiong X, Demianczuk NN, Saunders LD, Wang FL, Fraser WD. Impact of preeclampsia and gestational hypertension on birth weight by gestational age. Am J Epidemiol. 2002;155(3):203–9.
Dahlstrom B, Romundstad P, Oian P, Vatten LJ, Eskild A. Placenta weight in pre-eclampsia. Acta Obstet Gynecol Scand. 2008;87(6):608–11.
Caughey AB, Stotland NE, Escobar GJ. What is the best measure of maternal complications of term pregnancy: ongoing pregnancies or pregnancies delivered? Am J Obstet Gynecol. 2003;189(4):1047–52.
Burton GJ, Redman CW, Roberts JM, Moffett A. Pre-eclampsia: pathophysiology and clinical implications. BMJ. 2019;366:l2381.
Tobinaga CM, Torloni MR, Gueuvoghlanian-Silva BY, Pendeloski KP, Akita PA, Sass N, et al. Angiogenic factors and uterine Doppler velocimetry in early- and late-onset preeclampsia. Acta Obstet Gynecol Scand. 2014;93(5):469–76.
Soto E, Romero R, Kusanovic JP, Ogge G, Hussein Y, Yeo L, et al. Late-onset preeclampsia is associated with an imbalance of angiogenic and anti-angiogenic factors in patients with and without placental lesions consistent with maternal underperfusion. J Matern Fetal Neonatal Med. 2012;25(5):498–507.
Cho HY, Hwang HS, Jung I, Park YW, Kwon JY, Kim YH. Diagnosis of placenta accreta by uterine artery Doppler velocimetry in patients with placenta previa. J Ultrasound Med. 2015;34(9):1571–5.
Fahmy M, Alhalabi A, Hamza H, Abd ElZaher E, Abd El Fattah Khourshid Y, El Sabia M. Role of uterine artery Doppler in the diagnosis of placenta accrete in patients with placental previa. Menoufia Med J. 2020;33:497–500.
Kandil IM, Mohamed MA, Taha AM. Diagnosis of placenta accreta by uterine artery doppler velicometry in patients with placenta previa. Egypt J Hosp Med. 2019;77:5298–306.
Tantbirojn P, Crum CP, Parast MM. Pathophysiology of placenta creta: the role of decidua and extravillous trophoblast. Placenta. 2008;29(7):639–45.
Hannon T, Innes BA, Lash GE, Bulmer JN, Robson SC. Effects of local decidua on trophoblast invasion and spiral artery remodeling in focal placenta creta - an immunohistochemical study. Placenta. 2012;33(12):998–1004.
Khong TY, Robertson WB. Placenta creta and placenta praevia creta. Placenta. 1987;8(4):399–409.
Nakamura Y, Yaguchi C, Itoh H, Sakamoto R, Kimura T, Furuta N, et al. Morphologic characteristics of the placental basal plate in in vitro fertilization pregnancies: a possible association with the amount of bleeding in delivery. Hum Pathol. 2015;46(8):1171–9.
Jauniaux E, Hussein AM, Elbarmelgy RM, Elbarmelgy RA, Burton GJ. Failure of placental detachment in accreta placentation is associated with excessive fibrinoid deposition at the utero-placental interface. Am J Obstet Gynecol. 2022;226(2):243e1-e10.
Lim HJ, Sun J, Min B, Song M, Kim TH, Kim BJ, et al. Endometriosis and adverse pregnancy outcomes: a nationwide population-based study. J Clin Med. 2023;12(16):5392.
Zhang J, Liu X, Rao L, Ma R, Wu W, Chen C, et al. Adverse obstetric and perinatal outcomes of patients with history of recurrent miscarriage: a retrospective cohort study. Fertil Steril. 2023;120(3 Pt 2):626–34.
Conrad KP. G-Protein-coupled receptors as potential drug candidates in preeclampsia: targeting the relaxin/insulin-like family peptide receptor 1 for treatment and prevention. Hum Reprod Update. 2016;22(5):647–64.
Conrad KP, Shroff SG. Effects of relaxin on arterial dilation, remodeling, and mechanical properties. Curr Hypertens Rep. 2011;13(6):409–20.
Novak J, Danielson LA, Kerchner LJ, Sherwood OD, Ramirez RJ, Moalli PA, et al. Relaxin is essential for renal vasodilation during pregnancy in conscious rats. J Clin Invest. 2001;107(11):1469–75.
Debrah DO, Novak J, Matthews JE, Ramirez RJ, Shroff SG, Conrad KP. Relaxin is essential for systemic vasodilation and increased global arterial compliance during early pregnancy in conscious rats. Endocrinology. 2006;147(11):5126–31.
Conrad KP, Baker VL. Corpus luteal contribution to maternal pregnancy physiology and outcomes in assisted reproductive technologies. Am J Physiol Regul Integr Comp Physiol. 2013;304(2):R69-72.
von Versen-Hoynck F, Schaub AM, Chi YY, Chiu KH, Liu J, Lingis M, et al. Increased preeclampsia risk and reduced aortic compliance with in vitro fertilization cycles in the absence of a corpus luteum. Hypertension. 2019;73(3):640–9.
Conrad KP, Petersen JW, Chi YY, Zhai X, Li M, Chiu KH, et al. Maternal cardiovascular dysregulation during early pregnancy after in vitro fertilization cycles in the absence of a corpus luteum. Hypertension. 2019;74(3):705–15.
Conrad KP, Taher S, Chi YY, Qiu Y, Li M, Lingis M, et al. Relationships between reproductive hormones and maternal pregnancy physiology in women conceiving with or without in vitro fertilization. Am J Physiol Regul Integr Comp Physiol. 2021;321(3):R454–68.
Maynard SE, Min JY, Merchan J, Lim KH, Li J, Mondal S, et al. Excess placental soluble fms-like tyrosine kinase 1 (sFlt1) may contribute to endothelial dysfunction, hypertension, and proteinuria in preeclampsia. J Clin Invest. 2003;111(5):649–58.
Osol G, Celia G, Gokina N, Barron C, Chien E, Mandala M, et al. Placental growth factor is a potent vasodilator of rat and human resistance arteries. Am J Physiol Heart Circ Physiol. 2008;294(3):H1381–7.
Conrad KP, von Versen-Hoynck F, Baker VL. Potential role of the corpus luteum in maternal cardiovascular adaptation to pregnancy and preeclampsia. Am J Obstet Gynecol. 2022;226:683–99.
Yin R, Dang Y, Ma Z, Sun M. The effects of unexpected follicular growth and ovulation in artificial cycles: a retrospective cohort study of frozen, single-blastocyst transfer. Fertil Steril. 2023;119(6):985–93.
Gu F, Wu Y, Tan M, Hu R, Chen Y, Li X, et al. Programmed frozen embryo transfer cycle increased risk of hypertensive disorders of pregnancy: a multicenter cohort study in ovulatory women. Am J Obstet Gynecol MFM. 2023;5(1):100752.
Sacha CR, Harris AL, James K, Basnet K, Freret TS, Yeh J, et al. Placental pathology in live births conceived with in vitro fertilization after fresh and frozen embryo transfer. Am J Obstet Gynecol. 2020;222(4):360e1-e16.
Ogunleye O, Campo B, Herrera D, Post Uiterweer ED, Conrad KP. Relaxin confers cytotrophoblast protection from hypoxia-reoxygenation injury through the phosphatidylinositol 3-kinase-Akt/protein kinase B cell survival pathway. Am J Physiol Regul Integr Comp Physiol. 2017;312(4):R559–68.
Gellersen B, Brosens IA, Brosens JJ. Decidualization of the human endometrium: mechanisms, functions, and clinical perspectives. Semin Reprod Med. 2007;25(6):445–53.
Gellersen B, Brosens J. Cyclic AMP and progesterone receptor cross-talk in human endometrium: a decidualizing affair. J Endocrinol. 2003;178(3):357–72.
Lane B, Oxberry W, Mazella J, Tseng L. Decidualization of human endometrial stromal cells in vitro: effects of progestin and relaxin on the ultrastructure and production of decidual secretory proteins. Hum Reprod. 1994;9(2):259–66.
Tang M, Mazella J, Zhu HH, Tseng L. Ligand activated relaxin receptor increases the transcription of IGFBP-1 and prolactin in human decidual and endometrial stromal cells. Mol Hum Reprod. 2005;11(4):237–43.
Unemori EN, Erikson ME, Rocco SE, Sutherland KM, Parsell DA, Mak J, et al. Relaxin stimulates expression of vascular endothelial growth factor in normal human endometrial cells in vitro and is associated with menometrorrhagia in women. Hum Reprod. 1999;14(3):800–6.
Palejwala S, Tseng L, Wojtczuk A, Weiss G, Goldsmith LT. Relaxin gene and protein expression and its regulation of procollagenase and vascular endothelial growth factor in human endometrial cells. Biol Reprod. 2002;66(6):1743–8.
Tseng L, Zhu HH, Mazella J, Koistinen H, Seppala M. Relaxin stimulates glycodelin mRNA and protein concentrations in human endometrial glandular epithelial cells. Mol Hum Reprod. 1999;5(4):372–5.
Bartsch O, Bartlick B, Ivell R. Phosphodiesterase 4 inhibition synergizes with relaxin signaling to promote decidualization of human endometrial stromal cells. J Clin Endocrinol Metab. 2004;89(1):324–34.
Mazella J, Tang M, Tseng L. Disparate effects of relaxin and TGFbeta1: relaxin increases, but TGFbeta1 inhibits, the relaxin receptor and the production of IGFBP-1 in human endometrial stromal/decidual cells. Hum Reprod. 2004;19(7):1513–8.
Dimitriadis E, Stoikos C, Baca M, Fairlie WD, McCoubrie JE, Salamonsen LA. Relaxin and prostaglandin E(2) regulate interleukin 11 during human endometrial stromal cell decidualization. J Clin Endocrinol Metab. 2005;90(6):3458–65.
Yki-Jarvinen H, Wahlstrom T, Seppala M. Human endometrium contains relaxin that is progesterone-dependent. Acta Obstet Gynecol Scand. 1985;64(8):663–5.
Bryant-Greenwood GD, Rutanen EM, Partanen S, Coelho TK, Yamamoto SY. Sequential appearance of relaxin, prolactin and IGFBP-1 during growth and differentiation of the human endometrium. Mol Cell Endocrinol. 1993;95(1–2):23–9.
Stewart DR, Erikson MS, Erikson ME, Nakajima ST, Overstreet JW, Lasley BL, et al. The role of relaxin in glycodelin secretion. J Clin Endocrinol Metab. 1997;82(3):839–46.
Bond CP, Parry LJ, Samuel CS, Gehring HM, Lederman FL, Rogers PA, et al. Increased expression of the relaxin receptor (LGR7) in human endometrium during the secretory phase of the menstrual cycle. J Clin Endocrinol Metab. 2004;89(7):3477–85.
Goldsmith LT, Weiss G, Palejwala S, Plant TM, Wojtczuk A, Lambert WC, et al. Relaxin regulation of endometrial structure and function in the rhesus monkey. Proc Natl Acad Sci U S A. 2004;101:4685–9.
Vodstrcil LA, Tare M, Novak J, Dragomir N, Ramirez RJ, Wlodek ME, et al. Relaxin mediates uterine artery compliance during pregnancy and increases uterine blood flow. FASEB J. 2012;26(10):4035–44.
Anumba DO, El Gelany S, Elliott SL, Li TC. Serum relaxin levels are reduced in pregnant women with a history of recurrent miscarriage, and correlate with maternal uterine artery Doppler indices in first trimester. Eur J Obstet Gynecol Reprod Biol. 2009;147(1):41–5.
Jauniaux E, Johnson MR, Jurkovic D, Ramsay B, Campbell S, Meuris S. The role of relaxin in the development of the uteroplacental circulation in early pregnancy. Obstet Gynecol. 1994;84(3):338–42.
Moreno-Sepulveda J, Checa MA. Risk of adverse perinatal outcomes after oocyte donation: a systematic review and meta-analysis. J Assist Reprod Genet. 2019;36(10):2017–37.
Salha O, Sharma V, Dada T, Nugent D, Rutherford AJ, Tomlinson AJ, et al. The influence of donated gametes on the incidence of hypertensive disorders of pregnancy. Hum Reprod. 1999;14(9):2268–73.
Li N, Fan L, Cai H, Pan D, Shi W, Shi J, Wang H. Luteal phase support of intramuscular progesterone associated with lower hypertensive disorders of pregnancy as compared to vaginal progesterone: A cohort study. Int J Gynaecol Obstet. 2024. https://doi.org/10.1002/ijgo.15347
Sites CK, Wilson D, Barsky M, Bernson D, Bernstein IM, Boulet S, et al. Embryo cryopreservation and preeclampsia risk. Fertil Steril. 2017;108(5):784–90.
Xu J, Zhou H, Zhou T, Guo Y, Liang S, Jia Y, et al. The impact of different endometrial preparation protocols on obstetric and neonatal complications in frozen-thawed embryo transfer: a retrospective cohort study of 3,458 singleton deliveries. Reprod Biol Endocrinol. 2022;20(1):141.
Gui J, Ling Z, Hou X, Fan Y, Xie K, Shen R. In vitro fertilization is associated with the onset and progression of preeclampsia. Placenta. 2020;89:50–7.
Li L, Liu L, Xu Y. Hypertension in pregnancy as a risk factor for placenta accreta spectrum: a systematic review incorporating a network meta-analysis. Arch Gynecol Obstet. 2023;307(5):1323–9.
Zheng P, Chen D, Ye B, Yang X, Cheng W. Association between placental implantation abnormalities and hypertensive disorders of pregnancy. J Obstet Gynaecol Res. 2022;48(3):654–62.
Stanek J. Placenta creta: a spectrum of lesions associated with shallow placental implantation. Obstet Gynecol Int. 2020;2020:4230451.
Lloyd-Davies C, Collins SL, Burton GJ. Understanding the uterine artery Doppler waveform and its relationship to spiral artery remodelling. Placenta. 2021;105:78–84.
Niu Y, Suo L, Zhao D, Wang Y, Miao R, Zou J, et al. Is artificial endometrial preparation more associated with early-onset or late-onset preeclampsia after frozen embryo transfer? J Assist Reprod Genet. 2023;40(5):1045–54.
Wang Z, Zhang Y, Shang X, Miao R, Yin M, Yang H, et al. The likelihood of a healthy live birth after frozen embryo transfer with endometrium prepared by natural ovulation regimen vs programmed regimen: a propensity-score matching study. AJOG Glob Rep. 2023;3(2):100210.
Landsverk E, Westvik-Johari K, Romundstad LB, Opdahl S. Birth size after embryo cryopreservation: larger by all measures? Hum Reprod. 2023;38(7):1379–89.
Westvik-Johari K, Romundstad LB, Lawlor DA, Bergh C, Gissler M, Henningsen AA, et al. Separating parental and treatment contributions to perinatal health after fresh and frozen embryo transfer in assisted reproduction: a cohort study with within-sibship analysis. PLoS Med. 2021;18(6):e1003683.
Norwitz ER, Bonney EA, Snegovskikh VV, Williams MA, Phillippe M, Park JS, et al. Molecular regulation of parturition: the role of the decidual clock. Cold Spring Harb Perspect Med. 2015;5(11):a023143.
Redman CW, Sargent IL, Staff AC. IFPA Senior award lecture: making sense of pre-eclampsia - two placental causes of preeclampsia? Placenta. 2014;35(Suppl):S20–5.
McNally L, Zhou Y, Robinson JF, Zhao G, Chen LM, Chen H, et al. Up-regulated cytotrophoblast DOCK4 contributes to over-invasion in placenta accreta spectrum. Proc Natl Acad Sci U S A. 2020;117(27):15852–61.
Illsley NP, DaSilva-Arnold SC, Zamudio S, Alvarez M, Al-Khan A. Trophoblast invasion: lessons from abnormally invasive placenta (placenta accreta). Placenta. 2020;102:61–6.
Busnelli A, Schirripa I, Fedele F, Bulfoni A, Levi-Setti PE. Obstetric and perinatal outcomes following programmed compared to natural frozen-thawed embryo transfer cycles: a systematic review and meta-analysis. Hum Reprod. 2022;37(7):1619–41.
Zaat TR, Kostova EB, Korsen P, Showell MG, Mol F, van Wely M. Obstetric and neonatal outcomes after natural versus artificial cycle frozen embryo transfer and the role of luteal phase support: a systematic review and meta-analysis. Hum Reprod Update. 2023;29(5):634–54.
Moreno-Sepulveda J, Espinos JJ, Checa MA. Lower risk of adverse perinatal outcomes in natural versus artificial frozen-thawed embryo transfer cycles: a systematic review and meta-analysis. Reprod Biomed Online. 2021;42(6):1131–45.
Al-Khatib A, Sagot P, Cottenet J, Aroun M, Quantin C, Desplanches T. Major postpartum haemorrhage after frozen embryo transfer: a population-based study. BJOG. 2024;131(3):300–08.
Taniguchi M, Akinaga C, Suzuki K, Tarui K, Tamura N, Shiko Y, et al. The effect of assisted reproductive technology on postpartum bleeding: hormonal cycle frozen embryo transfer might increase blood loss. J Anesth. 2024;38(1):19–28.
Kaser DJ, Melamed A, Bormann CL, Myers DE, Missmer SA, Walsh BW, et al. Cryopreserved embryo transfer is an independent risk factor for placenta accreta. Fertil Steril. 2015;103(5):1176-84e2.
Ginstrom Ernstad E, Wennerholm UB, Khatibi A, Petzold M, Bergh C. Neonatal and maternal outcome after frozen embryo transfer: increased risks in programmed cycles. Am J Obstet Gynecol. 2019;221(2):1261–18.
Anim-Nyame N, Hills FA, Sooranna SR, Steer PJ, Johnson MR. A longitudinal study of maternal plasma insulin-like growth factor binding protein-1 concentrations during normal pregnancy and pregnancies complicated by pre-eclampsia. Hum Reprod. 2000;15(10):2215–9.
Siddiqui MF, Nandi P, Girish GV, Nygard K, Eastabrook G, de Vrijer B, et al. Decorin over-expression by decidual cells in preeclampsia: a potential blood biomarker. Am J Obstet Gynecol. 2016;215(3):361e1-e15.
Tamura I, Shiroshita A, Fujimura T, Tanaka-Doi Y, Shirafuta Y, Maekawa R, et al. Genome-wide analysis of histone modifications that underlie the dynamic changes in gene expression during decidualization in human endometrial stromal cells. Mol Hum Reprod. 2023;29(7):gaad019.
Garrido-Gomez T, Castillo-Marco N, Clemente-Ciscar M, Cordero T, Munoz-Blat I, Amadoz A, et al. Disrupted PGR-B and ESR1 signaling underlies defective decidualization linked to severe preeclampsia. Elife. 2021;10:e70753.
Burton GJ, Turco MY. Joan Hunt senior award lecture: new tools to shed light on the ‘black box’ of pregnancy. Placenta. 2022;125:54–60.
Baksh S, Casper A, Christianson MS, Devine K, Doody KJ, Ehrhardt S, et al. Natural vs. programmed cycles for frozen embryo transfer: study protocol for an investigator-initiated, randomized, controlled, multicenter clinical trial. Trials. 2021;22(1):660.
Liu X, Wen W, Wang T, Sun T, Wang T, Zhang N, et al. Comparison of endometrial preparation protocols (natural cycle versus hormone replacement cycle) for frozen embryo transfer (COMPETE): a study protocol for a randomised controlled trial. BMJ Open. 2022;12(10):e063981.
Funding
Investigations by KPC, VLB, and FVH were supported, in part, by a grant from the National Institutes of Health P01 HD065647: KPC Program Director/Principal Investigator, University of Florida; VLB Principal Investigator, Stanford University; FVH was also underwritten by a grant from the German Research Foundation DFG project number 273703678. VLB is currently supported by funding from the National Institutes of Health R01 HD100341 to study frozen embryo transfer protocols and FVH by DFG project number 507276351 to study decidualization.
Author information
Authors and Affiliations
Contributions
KPC conceived and drafted the review; KPC, FVH, and VLB revised, edited, and approved the final version.
Corresponding author
Ethics declarations
Ethical approval
Not applicable.
Consent to participate
Not applicable.
Consent for publication
The authors gave consent for publication.
Competing interests
KPC holds use patents for relaxin.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Dedication
We dedicate this work to Robert N. Taylor MD, PhD—colleague, friend and mentor. His exceptional intelligence, kindness, generosity and humility will never be forgotten.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Conrad, K.P., von Versen-Höynck, F. & Baker, V.L. Pathologic maternal and neonatal outcomes associated with programmed embryo transfer: potential etiologies and strategies for prevention. J Assist Reprod Genet 41, 843–859 (2024). https://doi.org/10.1007/s10815-024-03042-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10815-024-03042-8