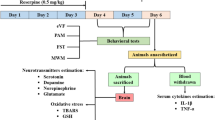
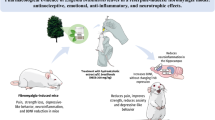
Abstract
Fibromyalgia is a potentially disabling idiopathic disease characterized by widespread chronic pain associated with comorbidities such as fatigue, anxiety, and depression. Current therapeutic approaches present adverse effects that limit adherence to therapy. Diosmetin, an aglycone of the flavonoid glycoside diosmin found in citrus fruits and the leaves of Olea europaea L., has antinociceptive, anti-inflammatory, and antioxidant properties. Here, we investigated the effect of diosmetin on nociceptive behaviors and comorbidities in an experimental fibromyalgia model induced by reserpine in mice. To induce the experimental fibromyalgia model, a protocol of subcutaneous injections of reserpine (1 mg/kg) was used once a day for three consecutive days in adult male Swiss mice. Mice received oral diosmetin on the fourth day after the first reserpine injection. Nociceptive (mechanical allodynia, muscle strength, and thermal hyperalgesia) and comorbid (depressive-like and anxiety behavior) parameters were evaluated. Potential adverse effects associated with diosmetin plus reserpine (locomotor alteration, cataleptic behavior, and body weight and temperature changes) were also evaluated. Oral diosmetin (0.015–1.5 mg/kg) reduced the mechanical allodynia, thermal hyperalgesia, and loss of muscle strength induced by reserpine. Diosmetin (0.15 mg/kg) also attenuated depressive-like and anxiety behaviors without causing locomotor alteration, cataleptic behavior, and alteration in weight and body temperature of mice. Overall, diosmetin can be an effective and safe therapeutic alternative to treat fibromyalgia symptoms, such as pain, depression and anxiety.



Similar content being viewed by others
Data availability
All data generated in this study are included in this manuscript.
Abbreviations
- B1:
-
Baseline 1
- B2:
-
Baseline 2
- DMSO:
-
Dimethylsulfoxide
- I max :
-
Maximum inhibition
- Nrf2:
-
Nuclear factor erythroid 2-related factor 2
- NF-κB:
-
Nuclear factor kappa B
- Keap1:
-
Kelch-like epichlorohydrin-associated protein 1
- p62:
-
Ubiquitin-binding protein p62
- PWT:
-
Paw withdrawal threshold
- SIRT1:
-
Silent information regulator 1
- TRPV1:
-
Transient potential vanilloid receptor subtype 1
References
Adamante G, de Almeida AS, Rigo FK et al (2019) Diosmetin as a novel transient receptor potential vanilloid 1 antagonist with antinociceptive activity in mice. Life Sci 216:215–226. https://doi.org/10.1016/j.lfs.2018.11.029
Arnold LM, Gebke KB, Choy EHS (2016) Fibromyalgia: Management strategies for primary care providers. Int J Clin Pract 70:99–112. https://doi.org/10.1111/IJCP.12757
Atwal N, Casey SL, Mitchell VA, Vaughan CW (2019) THC and gabapentin interactions in a mouse neuropathic pain model. Neuropharmacology 144:115–121. https://doi.org/10.1016/j.neuropharm.2018.10.006
Brederson J-D, Jarvis FM, Honore PS, Surowy C (2011) Fibromyalgia: mechanisms, current treatment and animal models. Curr Pharm Biotechnol 12:1613–1626. https://doi.org/10.2174/138920111798357258
Brum ES, Fialho MFP, Fischer SPM et al (2020) Relevance of Mitochondrial Dysfunction in the Reserpine-Induced Experimental Fibromyalgia Model. Mol Neurobiol 57(4202):4217. https://doi.org/10.1007/s12035-020-01996-1
Brum ES, Becker G, Fialho MFP, Oliveira SM (2022) Animal models of fibromyalgia: What is the best choice? Pharmacol Ther 230:107959. https://doi.org/10.1016/j.pharmthera.2021.107959
Brusco I, Justino AB, Silva CR et al (2019) Kinins and their B1 and B2 receptors are involved in fibromyalgia-like pain symptoms in mice. Biochem Pharmacol 168:119–132. https://doi.org/10.1016/j.bcp.2019.06.023
Brusco I, Justino AB, Silva CR et al (2021) Inhibitors of angiotensin I converting enzyme potentiate fibromyalgia-like pain symptoms via kinin receptors in mice. Eur J Pharmacol 895:173870. https://doi.org/10.1016/j.ejphar.2021.173870
Camponogara C, Brum ES, Pegoraro NS et al (2021) Diosmetin, a novel transient receptor potential vanilloid 1 antagonist, alleviates the UVB radiation-induced skin inflammation in mice. Inflammopharmacology 29:879–895. https://doi.org/10.1007/s10787-021-00802-1
Carballo-Villalobos AI, González-Trujano ME, Pellicer F et al (2018) Central and peripheral anti-hyperalgesic effects of diosmin in a neuropathic pain model in rats. Biomed Pharmacother 97:310–320. https://doi.org/10.1016/j.biopha.2017.10.077
Carradori S, Gidaro MC, Petzer A et al (2016) Inhibition of human monoamine oxidase: biological and molecular modeling studies on selected natural flavonoids. J Agric Food Chem 64:9004–9011. https://doi.org/10.1021/acs.jafc.6b03529
Chaplan SR, Bach FW, Pogrel JW et al (1994) Quantitative assessment of tactile allodynia in the rat paw. J Neurosci Methods 53:55–63. https://doi.org/10.1016/0165-0270(94)90144-9
Choy EHS (2015) The role of sleep in pain and fibromyalgia. Nat Rev Rheumatol 11:513–520
Choy E, Perrot S, Leon T et al (2010) A patient survey of the impact of fibromyalgia and the journey to diagnosis. BMC Health Serv Res 10:102. https://doi.org/10.1186/1472-6963-10-102
Clauw DJ (2014) Fibromyalgia. JAMA 311:1547. https://doi.org/10.1001/jama.2014.3266
Clauw DJ (2015) Fibromyalgia and related conditions. Mayo Clin Proc 90:680–692. https://doi.org/10.1016/j.mayocp.2015.03.014
De la Luz-Cuellar YE, Rodríguez-Palma EJ, Franco-Enzástiga Ú et al (2019) Blockade of spinal receptors differentially reduces reserpine-induced fibromyalgia-type pain in female rats. Eur J Pharmacol 858:1724443
de Oliveira DR, Todo AH, Rêgo GM et al (2018) Flavones-bound in benzodiazepine site on GABA A receptor: Concomitant anxiolytic-like and cognitive-enhancing effects produced by Isovitexin and 6-C-glycoside-Diosmetin. Eur J Pharmacol 831:77–86. https://doi.org/10.1016/j.ejphar.2018.05.004
Derry S, Cording M, Wiffen PJ et al (2016) Pregabalin for pain in fibromyalgia in adults. Cochrane Database Syst Rev 2019:291–292. https://doi.org/10.1002/14651858.CD011790.pub2
Dewanjee S, Hossain S, Ansari F (2023) Heliyon Review article A comprehensive review on clinically proven natural products in the management of nerve pain, with mechanistic insights. Heliyon 9:e15346. https://doi.org/10.1016/j.heliyon.2023.e15346
Dixon WJ (1980) Efficient analysis of experimental observations. Annu Rev Pharmacol Toxicol 20:441–462. https://doi.org/10.1146/annurev.pa.20.040180.002301
Doppler K, Rittner HL, Deckart M, Sommer C (2015) Reduced dermal nerve fiber diameter in skin biopsies of patients with fibromyalgia. Pain 156:2319–2325. https://doi.org/10.1097/j.pain.0000000000000285
Ferrarini EG, Paes RS, Baldasso GM et al (2022) Broad-spectrum cannabis oil ameliorates reserpine-induced fibromyalgia model in mice. Biomed Pharmacother 154:113552. https://doi.org/10.1016/j.biopha.2022.113552
Fialho MFP, Brum ES, Becker G et al (2023) Kinin B2 and B1 Receptors Activation Sensitize the TRPA1 Channel Contributing to Anastrozole-Induced Pain Symptoms. Pharmaceutics 15:1136. https://doi.org/10.3390/pharmaceutics15041136
Fischer SPM, Brusco I, Brum ES et al (2020) Involvement of TRPV1 and the efficacy of α-spinasterol on experimental fibromyalgia symptoms in mice. Neurochem Int 134:104673. https://doi.org/10.1016/j.neuint.2020.104673
Garcia Mendes MP, Carvalho dos Santos D, Rezende MJS et al (2021) Effects of intravenous administration of recombinant Phα1β toxin in a mouse model of fibromyalgia. Toxicon 195:104–110. https://doi.org/10.1016/j.toxicon.2021.03.012
Gavva NR, Treanor JJS, Garami A et al (2008) Pharmacological blockade of the vanilloid receptor TRPV1 elicits marked hyperthermia in humans. Pain 136:202–210. https://doi.org/10.1016/j.pain.2008.01.024
Gibson HE, Edwards JG, Page RS et al (2008) TRPV1 channels mediate long-term depression at synapses on hippocampal interneurons. Neuron 57:746–759. https://doi.org/10.1016/j.neuron.2007.12.027
Guo Y, Li D, Cen X et al (2022) Diosmetin protects against cardiac hypertrophy via p62/Keap1/Nrf2 signaling pathway. Oxid Med Cell Longev 2022:1–14. https://doi.org/10.1155/2022/8367997
Häuser W, Ablin J, Fitzcharles M-A et al (2015) Fibromyalgia. Nat Rev Dis Prim 1:15022. https://doi.org/10.1038/nrdp.2015.22
Hernandez-Leon A, De la Luz-Cuellar YE, Granados-Soto V et al (2018) Sex differences and estradiol involvement in hyperalgesia and allodynia in an experimental model of fibromyalgia. Horm Behav 97:39–46. https://doi.org/10.1016/j.yhbeh.2017.10.011
Huang W, Calvo M, Karu K et al (2013) A clinically relevant rodent model of the HIV antiretroviral drug stavudine induced painful peripheral neuropathy. Pain 154:560–575. https://doi.org/10.1016/j.pain.2012.12.023
Julius D (2013) TRP channels and pain. Annu Rev Cell Dev Biol 29:355–384. https://doi.org/10.1146/annurev-cellbio-101011-155833
Kaur A, Singh L, Singh N et al (2019) Ameliorative effect of imperatorin in chemically induced fibromyalgia: role of NMDA/NFkB mediated downstream signaling. Biochem Pharmacol 166:56–69. https://doi.org/10.1016/j.bcp.2019.05.012
Klein CP, Sperotto NDM, Maciel IS et al (2014) Effects of D-series resolvins on behavioral and neurochemical changes in a fibromyalgia-like model in mice. Neuropharmacology 86:57–66. https://doi.org/10.1016/j.neuropharm.2014.05.043
Kosek E, Cohen M, Baron R et al (2016) Do we need a third mechanistic descriptor for chronic pain states? Pain 157:1382–1386. https://doi.org/10.1097/j.pain.0000000000000507
Li S, Han J, Wang D, sheng, et al (2016) Echinocystic acid reduces reserpine-induced pain/depression dyad in mice. Metab Brain Dis 31:455–463. https://doi.org/10.1007/s11011-015-9786-6
Liao W, Ning Z, Chen L et al (2014) Intracellular antioxidant detoxifying effects of diosmetin on 2,2-azobis(2-amidinopropane) dihydrochloride (AAPH)-induced oxidative stress through inhibition of reactive oxygen species generation. J Agric Food Chem 62:8648–8654. https://doi.org/10.1021/jf502359x
Littlejohn G (2015) Neurogenic neuroinflammation in fibromyalgia and complex regional pain syndrome. Nat Rev Rheumatol 11:639–648. https://doi.org/10.1038/nrrheum.2015.100
Littlejohn G, Guymer E (2018) Neurogenic inflammation in fibromyalgia. Semin Immunopathol 40:291–300. https://doi.org/10.1007/s00281-018-0672-2
Lu Y, Zhu S, He Y et al (2019) Phytochemical profile and antidepressant effect of Ormosia henryi prain leaf ethanol extract. Int J Mol Sci 20:3396. https://doi.org/10.3390/ijms20143396
Macfarlane GJ, Kronisch C, Dean LE et al (2017) EULAR revised recommendations for the management of fibromyalgia. Ann Rheum Dis 76:318–328. https://doi.org/10.1136/annrheumdis-2016-209724
Mei Z, Du L, Liu X et al (2022) Diosmetin alleviated cerebral ischemia/reperfusion injury in vivo and in vitro by inhibiting oxidative stress via the SIRT1/Nrf2 signaling pathway. Food Funct 13:198–212. https://doi.org/10.1039/D1FO02579A
Mitra R, Nersesyan A, Pentland K et al (2022) Diosmin and its glycocalyx restorative and <scp>anti-inflammatory</scp> effects on injured blood vessels. FASEB J 36:e22630. https://doi.org/10.1096/fj.202200053RR
Nagakura Y, Oe T, Aoki T, Matsuoka N (2009) Biogenic amine depletion causes chronic muscular pain and tactile allodynia accompanied by depression: a putative animal model of fibromyalgia. Pain 146:26–33. https://doi.org/10.1016/j.pain.2009.05.024
Nagakura Y, Ohsaka N, Azuma R et al (2018) Monoamine system disruption induces functional somatic syndromes associated symptomatology in mice. Physiol Behav 194:505–514. https://doi.org/10.1016/j.physbeh.2018.07.007
Oe T, Tsukamoto M, Nagakura Y (2010) Reserpine causes biphasic nociceptive sensitivity alteration in conjunction with brain biogenic amine tones in rats. Neuroscience 169:1860–1871. https://doi.org/10.1016/j.neuroscience.2010.06.061
Patel K, Gadewar M, Tahilyani V, Patel DK (2013) A review on pharmacological and analytical aspects of diosmetin: A concise report. Chin J Integr Med 19:792–800. https://doi.org/10.1007/s11655-013-1595-3
Pedron C, Antunes FTT, Rebelo IN et al (2021) Phoneutria nigriventer Tx3-–3 peptide toxin reduces fibromyalgia symptoms in mice. Neuropeptides 85:102094. https://doi.org/10.1016/j.npep.2020.102094
Raja SN, Carr DB, Cohen M et al (2020) The revised international association for the study of pain definition of pain: concepts, challenges, and compromises. Pain 161:1976–1982. https://doi.org/10.1097/j.pain.0000000000001939
Rehm SE, Koroschetz J, Gockel U et al (2010) A cross-sectional survey of 3035 patients with fibromyalgia: subgroups of patients with typical comorbidities and sensory symptom profiles. Rheumatology 49:1146–1152. https://doi.org/10.1093/rheumatology/keq066
Sarzi-Puttini P, Giorgi V, Marotto D (2020) Atzeni F (2020) Fibromyalgia: an update on clinical characteristics, aetiopathogenesis and treatment. Nat Rev Rheumatol 1611(16):645–660. https://doi.org/10.1038/s41584-020-00506-w
Taguchi T, Katanosaka K, Yasui M et al (2015) Peripheral and spinal mechanisms of nociception in a rat reserpine-induced pain model. Pain 156:415–427. https://doi.org/10.1097/01.j.pain.0000460334.49525.5e
Terzian AL, Aguiar DC, Guimarães FS, Moreira FA (2009) Modulation of anxiety-like behaviour by Transient Receptor Potential Vanilloid Type 1 (TRPV1) channels located in the dorsolateral periaqueductal gray. Euro Neuropsychopharmacol 19(3):188–195. https://doi.org/10.1016/j.euroneuro.2008.11.004
Treede RD, Rief W, Barke A et al (2015) A classification of chronic pain for ICD-11. Pain 156:1003–1007. https://doi.org/10.1097/j.pain.0000000000000160
Yang Y, Gong X, Huang L et al (2017) Diosmetin exerts anti-oxidative, anti-inflammatory and anti-apoptotic effects to protect against endotoxin-induced acute hepatic failure in mice. Oncotarget 8:30723
Yao X, Li L, Kandhare A et al (2019) Attenuation of reserpine-induced fibromyalgia via ROS and serotonergic pathway modulation by fisetin a plant flavonoid polyphenol. Exp Ther Med 1343:1355
Zhao L, Tao X, Wang Q et al (2024) Diosmetin alleviates neuropathic pain by regulating the Keap1/Nrf2/NF-κB signaling pathway. Biomed Pharmacother 170:116067. https://doi.org/10.1016/j.biopha.2023.116067
Zimmermann M (1983) Ethical guidelines for investigations of experimental pain in conscious animals. Pain 16:109–110. https://doi.org/10.1016/0304-3959(83)90201-4
Acknowledgements
We thank the Program in Biological Sciences: Toxicological Biochemistry of the Federal University of Santa Maria, and we thank the Federal University of Santa Maria. We thank you for the financial support given by FAPERGS, CAPES/PROEX, and CNPq. We thank CNPq, PIBITI/CNPq, CAPES/PROEX and PROBIC/FAPERGS for their fellowship support.
Funding
Funding support for this project was provided in part by the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior—Brasil (CAPES)—Finance Code 001; by the Fundação de Amparo à Pesquisa do Estado do Rio Grande do Sul-FAPERGS (Grant #21/2551–0001966-2) (Brazil); and by the Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq). CAPES/Programa de Excelência Acadêmica (PROEX) (process #88881.844988/2023-01, Grant #1333/2023). This study is part of the National Institute of Science and Technology in 3D printing and Advanced Materials Applied to Human and Veterinary Health (INCT_3D-Saúde) funded by CNPq, Brazil (grant #406436/2022–3). S.M.O. and D.B.R. are recipient of fellowship from CNPq (Grants #309404/2023–1 and #307690/2021–0, respectively). M.F.P.F. is recipient of fellowship from CAPES/PROEX (process #88882.182170/2018–01), L.P.M. is recipient of fellowship from Programa Institucional de Bolsas de Iniciação Científica (PROBIC)/FAPERGS, and A.F. is recipient of fellowship from Programa Institucional de Bolsas de Iniciação em Desenvolvimento Tecnológico e Inovação (PIBITI)/CNPq. We thank CNPq, PIBITI/CNPq, CAPES/PROEX and PROBIC/FAPERGS for their fellowship support.
Author information
Authors and Affiliations
Contributions
Participated in research design: [Lara P. Marquezin; Maria Fernanda P. Fialho; Sara M. Oliveira]. Conducted experiments: [Lara P. Marquezin; Maria Fernanda P. Fialho; Amanda Favarin; Jéssica D. Lara; Michele M. Pillat]. Performed data analysis: [Lara P. Marquezin; Maria Fernanda P. Fialho; Denis B. Rosemberg; Sara M. Oliveira]. Wrote or contributed to the writing of the manuscript: [Lara P. Marquezin; Maria Fernanda P. Fialho; Amanda Favarin; Jéssica D. Lara; Michele M. Pillat; Denis B. Rosemberg; Sara M. Oliveira]. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interests
The authors have no relevant financial or non-financial interests to disclose. All authors reviewed the manuscript and agreed to the submission and publication of data in Inflammopharmacology.
Ethical approval
All procedures performed in studies involving animals were in accordance with the ethical standards of the institution at which the studies were conducted and ethical approval was obtained from Institutional Animal Care and Use Committee of the Federal University of Santa Maria (Process #9094220222).
Consent to participate
No applicable.
Consent to publish
Not applicable.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Marquezin, L.P., Fialho, M.F.P., Favarin, A. et al. Diosmetin attenuates fibromyalgia-like symptoms in a reserpine-induced model in mice. Inflammopharmacol (2024). https://doi.org/10.1007/s10787-024-01473-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10787-024-01473-4