Abstract
Background
The clinical efficacy and safety of intravenous immunoglobulin (IVIg) treatment for COVID-19 remain controversial. This study aimed to map the current status and gaps of available evidence, and conduct a meta-analysis to further investigate the benefit of IVIg in COVID-19 patients.
Methods
Electronic databases were searched for systematic reviews/meta-analyses (SR/MAs), primary studies with control groups, reporting on the use of IVIg in patients with COVID-19. A random-effects meta-analysis with subgroup analyses regarding study design and patient disease severity was performed. Our outcomes of interest determined by the evidence mapping, were mortality, length of hospitalization (days), length of intensive care unit (ICU) stay (days), number of patients requiring mechanical ventilation, and adverse events.
Results
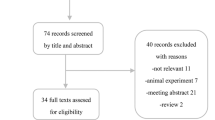
We included 34 studies (12 SR/MAs, 8 prospective and 14 retrospective studies). A total of 5571 hospitalized patients were involved in 22 primary studies. Random-effects meta-analyses of very low to moderate evidence showed that there was little or no difference between IVIg and standard care or placebo in reducing mortality (relative risk [RR] 0.91; 95% CI 0.78–1.06; risk difference [RD] 3.3% fewer), length of hospital (mean difference [MD] 0.37; 95% CI − 2.56, 3.31) and ICU (MD 0.36; 95% CI − 0.81, 1.53) stays, mechanical ventilation use (RR 0.92; 95% CI 0.68–1.24; RD 2.8% fewer), and adverse events (RR 0.98; 95% CI 0.84–1.14; RD 0.5% fewer) of patients with COVID-19. Sensitivity analysis using a fixed-effects model indicated that IVIg may reduce mortality (RR 0.76; 95% CI 0.60–0.97), and increase length of hospital stay (MD 0.68; 95% CI 0.09–1.28).
Conclusion
Very low to moderate certainty of evidence indicated IVIg may not improve the clinical outcomes of hospitalized patients with COVID-19. Given the discrepancy between the random- and fixed-effects model results, further large-scale and well-designed RCTs are warranted.







Similar content being viewed by others
Data availability
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
Aggarwal R, Dewan A, Pandey A, Trehan N, Majid MA (2022) Efficacy of high-dose intravenous immunoglobulin in severe and critical COVID-19: a retrospective cohort study. Int Immunopharmacol 106:108615. https://doi.org/10.1016/j.intimp.2022.108615
Ali HS, Elshafei MS, Saad MO, Mitwally HA, Al Wraidat M, Aroos A et al (2021a) Clinical outcomes of intravenous immunoglobulin therapy in COVID-19 related acute respiratory distress syndrome: a retrospective cohort study. BMC Pulm Med 21(1):354. https://doi.org/10.1186/s12890-021-01717-x
Ali S, Uddin SM, Shalim E, Sayeed MA, Anjum F, Saleem F et al (2021b) Hyperimmune anti-COVID-19 IVIG (C-IVIG) treatment in severe and critical COVID-19 patients: a phase I/II randomized control trial. EClinicalMedicine. https://doi.org/10.1016/j.eclinm.2021.100926
Arumugham VB, Rayi A (2022) Intravenous immunoglobulin (IVIG). StatPearls. StatPearls Publishing Copyright © 2022, StatPearls Publishing LLC, Treasure Island (FL)
Cao W, Liu X, Hong K, Ma Z, Zhang Y, Lin L et al (2021) High-dose intravenous immunoglobulin in severe coronavirus disease 2019: a multicenter retrospective study in China. Front Immunol 12:627844. https://doi.org/10.3389/fimmu.2021.627844
Chen Y, Xie J, Wu W, Li S, Hu Y, Hu M et al (2021a) Intravenous immunoglobulin therapy for critically Ill COVID-19 patients with different inflammatory phenotypes: a multicenter. Retrosp Study Front Immunol 12:738532. https://doi.org/10.3389/fimmu.2021.738532
Chen L, Zhang A, Li Q, Cui Y, Yuan G (2021b) Evaluation of clinical value of Xuebijing combined with human immunoglobulin in severe and critically ill patients with coronavirus disease 2019. Zhonghua Wei Zhong Bing Ji Jiu Yi Xue 33(4):399–404. https://doi.org/10.3760/cma.j.cn121430-20200628-00490
Cheng Q, Chen J, Jia Q, Fang Z, Zhao G (2021) Efficacy and safety of current medications for treating severe and non-severe COVID-19 patients: an updated network meta-analysis of randomized placebo-controlled trials. Aging (Albany NY) 13(18):21866–21902. https://doi.org/10.18632/aging.203522
Cheng Q, Zhao G, Chen J, Jia Q, Fang Z (2022) Comparative efficacy and safety of pharmacological interventions for severe COVID-19 patients: an updated network meta-analysis of 48 randomized controlled trials. Medicine (Baltimore) 101(41):e30998. https://doi.org/10.1097/md.0000000000030998
Dai C (2022) Application value of xuebijing combined with gamma globulin in corona virus disease 2019. China J Pharm Econ 17(07):65–8+72
Esen F, Özcan PE, Orhun G, Polat Ö, Anaklı İ, Alay G et al (2021) Effects of adjunct treatment with intravenous immunoglobulins on the course of severe COVID-19: results from a retrospective cohort study. Curr Med Res Opin 37(4):543–548. https://doi.org/10.1080/03007995.2020.1856058
Farrokhpour M, Rezaie N, Moradi N, Ghaffari Rad F, Izadi S, Azimi M et al (2021) Infliximab and intravenous gammaglobulin in hospitalized severe COVID-19 patients in intensive care unit. Arch Iran Med 24(2):139–143. https://doi.org/10.34172/aim.2021.22
Fei Z, Chen Z, Du X, Cao H, Li C (2022) Efficacy and safety of blood derivative therapy for patients with COVID-19: a systematic review and meta-analysis. Transfus Med Hemother 49(6):388–400. https://doi.org/10.1159/000524125
Ferrara G, Zumla A, Maeurer M (2012) Intravenous immunoglobulin (IVIg) for refractory and difficult-to-treat infections. Am J Med 125(10):1036.e1–8. https://doi.org/10.1016/j.amjmed.2012.01.023
Focosi D, Franchini M, Tuccori M, Cruciani M (2022) Efficacy of high-dose polyclonal intravenous immunoglobulin in COVID-19: a systematic review. Vaccines. https://doi.org/10.3390/vaccines10010094
Gharebaghi N, Nejadrahim R, Mousavi SJ, Sadat-Ebrahimi SR, Hajizadeh R (2020) The use of intravenous immunoglobulin gamma for the treatment of severe coronavirus disease 2019: a randomized placebo-controlled double-blind clinical trial. BMC Infect Dis 20(1):786. https://doi.org/10.1186/s12879-020-05507-4
Group TIIS (2022) Hyperimmune immunoglobulin for hospitalised patients with COVID-19 (ITAC): a double-blind, placebo-controlled, phase 3, randomised trial. Lancet (London, England) 399(10324):530–540. https://doi.org/10.1016/S0140-6736(22)00101-5
Higgins JP AD, Sterne JA (2017) Chapter 8: Assessing risk of bias in includedstudies. In: Higgins JP, Churchill R, Chandler J, Cumpston MS (eds) Cochrane Handbook for Systematic Reviews of Interventions Version 5.2.0. Cochrane 2017. www.training.cochrane.org/handbook/archive/v5.2. Accessed June 2017
Hou X, Tian L, Zhou L, Jia X, Kong L, Xue Y et al (2021) Intravenous immunoglobulin-based adjuvant therapy for severe COVID-19: a single-center retrospective cohort study. Virol J 18(1):101. https://doi.org/10.1186/s12985-021-01575-3
Huang C, Wang Y, Li X, Ren L, Zhao J, Hu Y et al (2020) Clinical features of patients infected with 2019 novel coronavirus in Wuhan. China Lancet 395(10223):497–506. https://doi.org/10.1016/s0140-6736(20)30183-5
Huang C, Fei L, Li W, Xu W, Xie X, Li Q et al (2021) Efficacy evaluation of intravenous immunoglobulin in non-severe patients with COVID-19: a retrospective cohort study based on propensity score matching. Int J Infect Dis 105:525–531. https://doi.org/10.1016/j.ijid.2021.01.009
Juul S, Nielsen EE, Feinberg J, Siddiqui F, Jørgensen CK, Barot E et al (2021) Interventions for treatment of COVID-19: second edition of a living systematic review with meta-analyses and trial sequential analyses (The LIVING Project). PloS One 16(3):e0248132. https://doi.org/10.1371/journal.pone.0248132
Kim MS, An MH, Kim WJ, Hwang TH (2020) Comparative efficacy and safety of pharmacological interventions for the treatment of COVID-19: a systematic review and network meta-analysis. PLoS Med 17(12):e1003501. https://doi.org/10.1371/journal.pmed.1003501
Krynytska I, Marushchak M, Birchenko I, Dovgalyuk A, Tokarskyy O (2021) COVID-19-associated acute respiratory distress syndrome versus classical acute respiratory distress syndrome (a narrative review). Iran J Microbiol 13(6):737–747. https://doi.org/10.18502/ijm.v13i6.8072
Lai CC, Chen WC, Chen CY, Wei YF (2022) The effect of intravenous immunoglobulins on the outcomes of patients with COVID-19: a systematic review and meta-analysis of randomized controlled trials. Expert Rev Anti Infect Ther 20(10):1333–1340. https://doi.org/10.1080/14787210.2022.2098112
Li Y, Qin Y, Chen N, Ge L, Wang Q, Aboudou T et al (2023) Use of traditional Chinese medicine for the treatment and prevention of COVID-19 and rehabilitation of COVID-19 patients: an evidence mapping study. Front Pharmacol 14:1069879. https://doi.org/10.3389/fphar.2023.1069879
Liu J, Chen Y, Li R, Wu Z, Xu Q, Li Z et al (2021) Intravenous immunoglobulin treatment for patients with severe COVID-19: a retrospective multicentre study. Clin Microbiol Infect 27(10):1488–1493. https://doi.org/10.1016/j.cmi.2021.05.012
Marcec R, Dodig VM, Radanovic I, Likic R (2022) Intravenous immunoglobulin (IVIg) therapy in hospitalised adult COVID-19 patients: a systematic review and meta-analysis. Rev Med Virol 32(6):e2397. https://doi.org/10.1002/rmv.2397
Mazeraud A, Jamme M, Mancusi RL, Latroche C, Megarbane B, Siami S et al (2022) Intravenous immunoglobulins in patients with COVID-19-associated moderate-to-severe acute respiratory distress syndrome (ICAR): multicentre, double-blind, placebo-controlled, phase 3 trial. Lancet Respir Med 10(2):158–166. https://doi.org/10.1016/S2213-2600(21)00440-9
Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD et al (2021) The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ 372:n71. https://doi.org/10.1136/bmj.n71
Pei L, Zhang S, Huang L, Geng X, Ma L, Jiang W et al (2020) Antiviral agents, glucocorticoids, antibiotics, and intravenous immunoglobulin in 1142 patients with coronavirus disease 2019: a systematic review and meta-analysis. Pol Arch Intern Med 130(9):726–733. https://doi.org/10.20452/pamw.15543
Rahmel T, Kraft F, Haberl H, Achtzehn U, Brandenburger T, Neb H et al (2022) Intravenous IgM-enriched immunoglobulins in critical COVID-19: a multicentre propensity-weighted cohort study. Crit Care 26(1):204. https://doi.org/10.1186/s13054-022-04059-0
Raman RS, Bhagwan Barge V, Anil Kumar D, Dandu H, Rakesh Kartha R, Bafna V et al (2021) A phase II safety and efficacy study on prognosis of moderate pneumonia in coronavirus disease 2019 patients with regular intravenous immunoglobulin therapy. J Infect Dis 223(9):1538–1543. https://doi.org/10.1093/infdis/jiab098
Review Manager (2020) Version 5.4: Copenhagen: The Cochrane Collaboration.
Romero C, Díez JM, Gajardo R (2022) Anti-SARS-CoV-2 antibodies in healthy donor plasma pools and IVIG products-an update. Lancet Infect Dis 22(1):19. https://doi.org/10.1016/s1473-3099(21)00755-6
Sakoulas G, Geriak M, Kullar R, Greenwood K, Habib M, Vyas A et al (2020) Use of intravenous immunoglobulin therapy reduces progression to mechanical ventilation in COVID-19 patients with moderate to severe hypoxia. Open Forum Infect Dis 7(Suppl 1):S166. https://doi.org/10.1093/ofid/ofaa417.381
Salehi M, Barkhori Mehni M, Akbarian M, Fattah Ghazi S, Khajavi Rad N, Moradi Moghaddam O et al (2022) The outcome of using intravenous immunoglobulin (IVIG) in critically ill COVID-19 patients’: a retrospective, multi-centric cohort study. Eur J Med Res 27(1):18. https://doi.org/10.1186/s40001-022-00637-8
Schünemann HJ, Vist GE, Glasziou P, Akl EA, Skoetz N (2022) Chapter 14:Completing ‘Summary of findings’ tables and grading the certainty of the evidence. In: Higgins JPT, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA (eds) Cochrane Handbook for Systematic Reviews of Interventions version 6.3 (updated February 2022). Cochrane 2022. www.training.cochrane.org/handbook.
Shao Z, Feng Y, Zhong L, Xie Q, Lei M, Liu Z et al (2020) Clinical efficacy of intravenous immunoglobulin therapy in critical ill patients with COVID-19: a multicenter retrospective cohort study. Clin Transl Immunol. https://doi.org/10.1002/cti2.1192
Shea BJ, Reeves BC, Wells G, Thuku M, Hamel C, Moran J et al (2017) AMSTAR 2: a critical appraisal tool for systematic reviews that include randomised or non-randomised studies of healthcare interventions, or both. BMJ 358:j4008. https://doi.org/10.1136/bmj.j4008
Siemieniuk RA, Bartoszko JJ, Díaz Martinez JP, Kum E, Qasim A, Zeraatkar D et al (2021) Antibody and cellular therapies for treatment of covid-19: a living systematic review and network meta-analysis. BMJ 374:n2231. https://doi.org/10.1136/bmj.n2231
Stang A (2010) Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur J Epidemiol 25(9):603–605. https://doi.org/10.1007/s10654-010-9491-z
Sun X, Wang T, Cai D, Hu Z, Chen J, Liao H et al (2020) Cytokine storm intervention in the early stages of COVID-19 pneumonia. Cytokine Growth Factor Rev 53:38–42. https://doi.org/10.1016/j.cytogfr.2020.04.002
Tabarsi P, Barati S, Jamaati H, Haseli S, Marjani M, Moniri A et al (2021) Evaluating the effects of Intravenous Immunoglobulin (IVIg) on the management of severe COVID-19 cases: a randomized controlled trial. Int Immunopharmacol 90:107205. https://doi.org/10.1016/j.intimp.2020.107205
Wang F, Yin Z, Jiang J, Shen G (2020) The effect of intravenous immunoglobulin on prognosis of critically ill patients with Coronavirus Disease 2019: a retrospective study. Chin J Hosp Pharm 40(14):1505–1510. https://doi.org/10.13286/j.1001-5213.2020.14.02, http://journal18.magtechjournal.com/Jwk_zgyyyx/EN/10.13286/j.1001-5213.2020.14.02
World Health Organization. Coronavirus disease (COVID-19) pandemic. https://www.who.int/emergencies/diseases/novel-coronavirus-2019.
Xiang HR, Cheng X, Li Y, Luo WW, Zhang QZ, Peng WX (2021) Efficacy of IVIG (intravenous immunoglobulin) for corona virus disease 2019 (COVID-19): A meta-analysis. Int Immunopharmacol 96:107732. https://doi.org/10.1016/j.intimp.2021.107732
Yaqinuddin A, Ambia AR, Elgazzar TA, AlSaud MBM, Kashir J (2021) Application of intravenous immunoglobulin (IVIG) to modulate inflammation in critical COVID-19 - a theoretical perspective. Med Hypotheses 151:110592. https://doi.org/10.1016/j.mehy.2021.110592
Zeng L, Brignardello-Petersen R, Hultcrantz M, Siemieniuk RAC, Santesso N, Traversy G et al (2021) GRADE guidelines 32: GRADE offers guidance on choosing targets of GRADE certainty of evidence ratings. J Clin Epidemiol 137:163–175. https://doi.org/10.1016/j.jclinepi.2021.03.026
Zhang C, Jin H, Wen YF, Yin G (2021) Efficacy of COVID-19 treatments: a bayesian network meta-analysis of randomized controlled trials. Front Public Health 9:729559. https://doi.org/10.3389/fpubh.2021.729559
Funding
This research was supported by the Science and technology Project of “Gansu Prescription” Prevention and Treatment of COVID-19 (Project No. 22ZD1FA001). The funding source had no role in the study design, data collection and analysis, decision to publish, or manuscript preparation.
Author information
Authors and Affiliations
Contributions
KY, ML, YL, and YW: conceived and designed the study. ML, YL: designed and performed the search strategy. ML, YL, ML, KG, MM, XW, NM, DL, ZL, LG, and XW: screened and selected the articles, extracted the data and assessed the risk of bias. ML and XX: analysed the data. LY and KY: supervised the data analysis. ML and XX: rated the certainty of evidence. LY and KY provided methodological support. ML, YL, XX, JN and KY: interpreted the data. ML and YL: drafted the manuscript. JJ, ZZ, BP, WS, ZS, YW, and KY: revised the manuscript, all authors reviewed the manuscript and approved the final version of the manuscript.
Corresponding authors
Ethics declarations
Conflict of interest
None of the authors have conflicts of interest associated with this manuscript to declare.
Ethical approval
This is a systematic review and meta-analysis study. Evidence-Based Medicine Center, School of Basic Medical Sciences of Lanzhou University has confirmed that no ethical approval is required.
Consent to participate
Not applicable.
Consent to publish
Not applicable.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Li, Mx., Li, Yf., Xing, X. et al. Intravenous immunoglobulin for treatment of hospitalized COVID-19 patients: an evidence mapping and meta-analysis. Inflammopharmacol 32, 335–354 (2024). https://doi.org/10.1007/s10787-023-01398-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10787-023-01398-4