Abstract
Background
Effort to search for the optimal COVID-19 treatment has continuously been attempted. Thymosin alpha-1 have immunomodulatory properties which may be beneficial in case of viral infection. This study’s goal is to determine whether thymosin alpha-1 is effective in treating people with moderate-to-severe COVID-19.
Methods
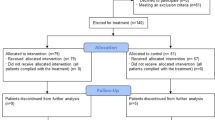
We searched for literature in 4 database: Scopus, Europe PMC, Medline, ClinicalTrials.gov, and Cochrane Library until March 25th, 2023. If those articles have data on the efficacy of thymosin alpha-1 therapy on COVID-19, they would be included. Risk ratio (RR) and Mean Difference (MD) along with their 95% confidence intervals were used to pool the results of dichotomous and continuous variables, respectively.
Results
Pooled data from 8 studies indicated that moderate to critical Covid-19 patients who were receiving thymosin alpha-1 therapy had significantly lower mortality from COVID-19 (RR 0.59; 95% CI 0.37–0.93, p = 0.02, I2 = 84%), but without any difference in the needs for mechanical ventilation (RR 0.83; 95% CI 0.48–1.44, p = 0.51, I2 = 74%) and hospital length of stay (MD 2.32; 95% CI – 0.93, 5.58, p = 0.16, I2 = 94%) compared to placebo. The benefits of thymosin alpha-1 on the mortality rate were significantly affected only by sample size (p = 0.0000) and sex (p = 0.0117).
Conclusion
Our study suggests that treatment with thymosin alpha-1 may reduce mortality rate in moderate to critical COVID-19 patients. Randomized clinical trials (RCTs) are still required to verify the findings of our study.


Similar content being viewed by others
Data availability
The datasets used and/or analyzed during the current study are available as RevMan file (.rm5) from the corresponding author on reasonable request.
References
Dominari A, Hathaway Iii D, Pandav K, Matos W, Biswas S, Reddy G et al (2020) Thymosin alpha 1: a comprehensive review of the literature. World J Virol 9(5):67–78. https://doi.org/10.5501/wjv.v9.i5.67
Higgins JP, Thompson SG, Deeks JJ, Altman DG (2003) Measuring inconsistency in meta-analyses. BMJ 327(7414):557–560. https://doi.org/10.1136/bmj.327.7414.557
Ivan Hariyanto T, Kurniawan A (2021) Tocilizumab administration is associated with the reduction in biomarkers of coronavirus disease 2019 infection. J Med Virol 93(3):1832–1836. https://doi.org/10.1002/jmv.26698
Kharazmi-Khorassani J, Asoodeh A, Tanzadehpanah H (2019) Antioxidant and angiotensin-converting enzyme (ACE) inhibitory activity of thymosin alpha-1 (Thα1) peptide. Bioorg Chem 87:743–752. https://doi.org/10.1016/j.bioorg.2019.04.003
Li J, Liu CH, Wang FS (2010) Thymosin alpha 1: biological activities, applications and genetic engineering production. Peptides 31(11):2151–2158. https://doi.org/10.1016/j.peptides.2010.07.026
Li Y, Li J, Ke J, Cheng S, Zhu L, Shen L (2020) Higher survival rate associated with thymosin alpha 1 use in critical COVID-19: a retrospective multicenter cohort study. Res Sq. https://doi.org/10.21203/rs.3.rs-123265/v1
Limen RY, Sedono R, Sugiarto A, Hariyanto TI (2022) Janus kinase (JAK)-inhibitors and coronavirus disease 2019 (COVID-19) outcomes: a systematic review and meta-analysis. Expert Rev Anti Infect Ther 20(3):425–434. https://doi.org/10.1080/14787210.2021.1982695
Liu Y, Pan Y, Hu Z, Wu M, Wang C, Feng Z et al (2020) Thymosin alpha 1 reduces the mortality of severe coronavirus disease 2019 by restoration of lymphocytopenia and reversion of exhausted T cells. Clin Infect Dis 71(16):2150–2157. https://doi.org/10.1093/cid/ciaa630
Liu J, Shen Y, Wen Z, Xu Q, Wu Z, Feng H et al (2021) Efficacy of thymosin alpha 1 in the treatment of COVID-19: a multicenter cohort study. Front Immunol 2(12):673693. https://doi.org/10.3389/fimmu.2021.673693
Liu T, Liu S, Li T, Zhang J (2022) Thymosin a1 use is not associated with reduced COVID-19 mortality. Am J Emerg Med 53:252–253. https://doi.org/10.1016/j.ajem.2021.04.043
Margulis AV, Pladevall M, Riera-Guardia N, Varas-Lorenzo C, Hazell L, Berkman ND et al (2014) Quality assessment of observational studies in a drug-safety systematic review, comparison of two tools: the Newcastle–Ottawa scale and the RTI item bank. Clin Epidemiol 10(6):359–368. https://doi.org/10.2147/CLEP.S66677
Matteucci C, Minutolo A, Balestrieri E, Petrone V, Fanelli M, Malagnino V et al (2020) Thymosin alpha 1 mitigates cytokine storm in blood cells from coronavirus disease 2019 patients. Open Forum Infect Dis 8(1):ofaa588. https://doi.org/10.1093/ofid/ofaa588
Mustafa H, Hamza A, Shah NN, Samreen S, Din W (2022) Evaluation of role of thymosin alpha 1 in moderate to severe COVID-19 patients: a retrospective study. Eur J Mol Clin Med 9(3):11870–11876
Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD et al (2021) The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ 29(372):n71. https://doi.org/10.1136/bmj.n71
Shehadeh F, Benitez G, Mylona EK, Tran QL, Tsikala-Vafea M, Atalla E et al (2022) A pilot trial of thymalfasin (Tα1) to treat hospitalized patients with hypoxemia and lymphocytopenia due to COVID-19 infection. J Infect Dis 3:jiac362. https://doi.org/10.1093/infdis/jiac362
Shetty A, Chandrakant NS, Darnule RA, Manjunath BG, Sathe P (2022) A double-blind multicenter two-arm randomized placebo-controlled phase-III clinical study to evaluate the effectiveness and safety of thymosin α1 as an add-on treatment to existing standard of care treatment in moderate-to-severe COVID-19 patients. Indian J Crit Care Med 26(8):913–919. https://doi.org/10.5005/jp-journals-10071-24298
Sterne JAC, Savović J, Page MJ, Elbers RG, Blencowe NS, Boutron I et al (2019) RoB 2: a revised tool for assessing risk of bias in randomised trials. BMJ 28(366):l4898. https://doi.org/10.1136/bmj.l4898
Stroup DF, Berlin JA, Morton SC, Olkin I, Williamson GD, Rennie D et al (2000) Meta-analysis of observational studies in epidemiology: a proposal for reporting. Meta-analysis of observational studies in epidemiology (MOOSE) group. JAMA 283(15):2008–2012. https://doi.org/10.1001/jama.283.15.2008
Sun Q, Xie J, Zheng R, Li X, Chen H, Tong Z et al (2021) The effect of thymosin α1 on mortality of critical COVID-19 patients: a multicenter retrospective study. Int Immunopharmacol 90:107143. https://doi.org/10.1016/j.intimp.2020.107143
Tao N, Xu X, Ying Y, Hu S, Sun Q, Lv G et al (2023) Thymosin α1 and its role in viral infectious diseases: the mechanism and clinical application. Molecules 28(8):3539. https://doi.org/10.3390/molecules28083539
Terrin N, Schmid CH, Lau J, Olkin I (2003) Adjusting for publication bias in the presence of heterogeneity. Stat Med 22(13):2113–2126. https://doi.org/10.1002/sim.1461
The Lancet (2023) The COVID-19 pandemic in 2023: far from over. Lancet 401(10371):79. https://doi.org/10.1016/S0140-6736(23)00050-8
Thornton A, Lee P (2000) Publication bias in meta-analysis: its causes and consequences. J Clin Epidemiol 53(2):207–216. https://doi.org/10.1016/s0895-4356(99)00161-4
van Paassen J, Vos JS, Hoekstra EM, Neumann KMI, Boot PC, Arbous SM (2020) Corticosteroid use in COVID-19 patients: a systematic review and meta-analysis on clinical outcomes. Crit Care 24(1):696. https://doi.org/10.1186/s13054-020-03400-9
Wang Z, Chen J, Zhu C, Liu L, Qi T, Shen Y et al (2021) Thymosin Alpha-1 has no beneficial effect on restoring CD4+ and CD8+ T lymphocyte counts in COVID-19 patients. Front Immunol 3(12):568789. https://doi.org/10.3389/fimmu.2021.568789
World Health Organization. Weekly epidemiological update on COVID-19 - 27 April 2023. Available from: https://www.who.int/publications/m/item/weekly-epidemiological-update-on-covid-19---27-april-2023
Wu M, Ji JJ, Zhong L, Shao ZY, Xie QF, Liu ZY et al (2020) Thymosin α1 therapy in critically ill patients with COVID-19: A multicenter retrospective cohort study. Int Immunopharmacol 88:106873. https://doi.org/10.1016/j.intimp.2020.106873
Zhang YH, Wang WY, Pang XC, Wang Z, Wang CZ, Zhou H et al (2022) Thymosin-α1 binds with ACE and downregulates the expression of ACE2 in human respiratory epithelia. Front Biosci (landmark Ed). 27(2):48. https://doi.org/10.31083/j.fbl2702048
Funding
The authors declare that no funds, grants, or other support were received during the preparation of this manuscript.
Author information
Authors and Affiliations
Contributions
Conceptualization: AYS, HS, TAY, TIH; Methodology: AYS and TAY; Formal analysis: AYS and TAY; Data curation: AYS and TAY, Validation: HS and TIH; Supervision: HS and TIH; Writing-original draft: AYS and TAY; Writing-review and editing: HS and TIH.
Corresponding author
Ethics declarations
Conflict of interest
The authors have no relevant financial or non-financial interests to disclose.
Ethical approval
This is a systematic review and meta-analysis study. The Faculty of Medicine, Pelita Harapan University Research Ethics Committee has confirmed that no ethical approval is required.
Consent to participate
Not applicable.
Consent to publish
Not applicable.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Soeroto, A.Y., Suryadinata, H., Yanto, T.A. et al. The efficacy of thymosin alpha-1 therapy in moderate to critical COVID-19 patients: a systematic review, meta-analysis, and meta-regression. Inflammopharmacol 31, 3317–3325 (2023). https://doi.org/10.1007/s10787-023-01354-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10787-023-01354-2