Abstract
The clinical drugs for ulcerative colitis mainly affect the inflammatory symposiums with limited outcomes and various side effects. Repairing the damaged intestinal mucosa is a promising and alternative strategy to treat ulcerative colitis. Trefoil factor family 2 (TFF2) could repair the intestinal mucosa, however, it has a short half-life in vivo. To improve the stability of TFF2, we have prepared a new fusion protein TFF2-Fc with much stability, investigated the therapeutic effect of TFF2-Fc on ulcerative colitis, and further illustrated the related mechanisms. We found that intrarectally administered TFF2-Fc alleviated the weight loss, the colon shortening, the disease activity index, the intestinal tissue injury, and the lymphocyte infiltration in dextran sulfate sodium (DSS)-induced colitis mice. In vitro, TFF2-Fc inhibited Caco2 cells injury and apoptosis, promoted cellular migration, and increased the expression of Occludin and ZO-1 by activating P-ERK in the presence of H2O2 or inflammatory conditioned medium (LPS-RAW264.7/CM). Moreover, TFF2-Fc could reduce lipopolysaccharide (LPS)-induced production of inflammation cytokines and reactive oxygen species in RAW264.7 cells, and also inhibits the polarization of RAW264.7 cells to M1 phenotype by reducing glucose consumption and lactate production. Taken together, in this work, we have prepared a novel fusion protein TFF2-Fc, which could alleviate ulcerative colitis in vivo via promoting intestinal epithelial cells repair and inhibiting macrophage inflammation, and TFF2-Fc might serve as a promising ulcerative colitis therapeutic agent.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Ulcerative colitis is a chronic relapsing disease characterized by bloody diarrhea and abdominal pain (Pagano et al. 2019). The pathogenesis of ulcerative colitis is still unclear (Liu et al. 2022), however, many evidences have proved that both intestinal mucosal injury and immune system imbalance are the main causes of ulcerative colitis (Neurath 2014). Therefore, the main therapeutic strategies of ulcerative colitis are to repair the damaged intestinal mucosa and/or suppress inflammation. The clinical drugs used to treat ulcerative colitis mainly focus on reducing the inflammatory response. Traditional chemical drugs including aminosalicylic acid, glucocorticoids, and sterols, could reduce the inflammatory symptoms in ulcerative colitis patients, but could not cure the disease completely (Feuerstein et al. 2019). Biological therapies, mainly including tumor necrosis factor-α (TNF-α) inhibitor infliximab, and integrin antagonist vedolizumab, could shorten the course of ulcerative colitis. However, infliximab is ineffective in two-thirds of patients and might increase the risk of cancer (Na and Moon 2019). Many novel biological active substances were also employed to treat ulcerative colitis by reducing inflammation, such as fish oil (Silvestri et al. 2020), plant cannabinoids (Pagano et al. 2021), and aqueous extract from ipomoea asarifolia (Da Silva et al. 2018). In addition, it has been reported that intestinal microbes could maintain host immune homeostasis and the stability and diversity of intestinal microbes could be protected by gerobiotics that include probiotics or derived postbiotics, and thus alleviate the inflammatory bowel disease (Agagunduz et al. 2022a, b). It should be noted that mucosal healing and epithelial cell repair could reduce the recurrence rate of ulcerative colitis (Boal Carvalho and Cotter 2017). Therefore, in addition to relieving inflammation and regulating the immune system, repairing damaged intestinal mucosa is also an effective strategy for treating ulcerative colitis (Wang et al. 2019). It has been reported that tofacitinib (a small-molecule pan-Janus kinase inhibitor) could restore mucosal damage, and thus inhibit DSS-induced colitis symptoms in colitis mice (Spalinger et al. 2021). Interleukin-33 (IL-33) promoted epithelial repair and recovery, and alleviated DSS-induced acute colitis in mice (Lopetuso et al. 2018). Therefore, it is promising to develop new drugs by repairing the damaged intestinal mucosa in ulcerative colitis.
Trefoil factor family (TFFs) consists of three small regulatory proteins (12−22 kDa), which could repair damaged gastrointestinal mucosa in vivo. TFF1 is mainly secreted in gastric pit cells, which could protect gastric mucosa and repair gastric ulcers (Jahan et al. 2020). TFF3 is mainly produced by mucus-secreting cells in the small intestine and colon, exogenous TFF3 could alleviate gastrointestinal mucosal damage in vivo (Podolsky et al. 2009). TFF2 is mainly expressed in epithelial cells deep in gastric glands, after gastrointestinal mucosal injury, TFF2 secretion increases in ulcer-associated cell lineage and plays a repairing role in the damaged area (Wong et al. 1999). Moreover, exogenous TFF2 could promote gastrointestinal cells, corneal epithelial cells, and bronchial epithelial cells to the damaged sites (Oertel et al. 2001), and thus promote mucosal repair and reduce the level of inflammatory factors (Tran et al. 1999). These studies strongly suggested that TFF2 could act as a potential therapeutic agent in the treatment of ulcerative colitis. However, the short half-life (48 min) in vivo limited the application of TFF2 as a therapeutic agent directly (Kjellev et al. 2007).
Generally, the fragment crystallizable (Fc) fusion protein could prolong the half-life of the functional protein (Wang et al. 2018). For example, wild-type fibroblast growth factor 21 (FGF21) has a very short half-life (1.5 h) in mice, while the half-life of Fc-FGF21 was 30.3 h (Pan et al. 2021). The half-life of thymosin alpha1 (Tα1)-Fc in circulation was 29 h, causing a nearly 14-fold increase compared with Tα1 (1.67 h) (Shen et al. 2018). Therefore, in the present study, TFF2-Fc fusion protein was prepared by recombinant technology to improve the stability of TFF2. We further investigated the therapeutic effect of TFF2-Fc on ulcerative colitis mice, illustrated the mechanisms of TFF2-Fc in promoting the repair of intestinal epithelial cells, and evaluated the anti-inflammatory effect in macrophages.
Methods and materials
Reagents and drugs
Lipopolysaccharide (LPS, L2880) and U0126 were purchased from Sigma-Aldrich (USA). Antibodies against ERK1/2, phosphorylated ERK1/2 (T202/Y204), GAPDH, Occludin, and ZO-1 were purchased from Cell Signaling Technology (Boston, MA, USA), Huaan Biotechnology Co., Ltd. (Hangzhou, China), and Wanleibio (Shenyang, China), respectively. DSS was purchased from APExBIO Technology LLC (Houston, TX, USA). Sulfasalazine (SASP) was obtained from MedChemexpress CO., Ltd. (NJ, USA). Glucose content detection kit was obtained from Solarbio (BC2500, Beijing, China), and lactic acid test kit was obtained from Nanjing Jiancheng Bioengineering Institute (A019-2-1, China).
Animals
Male C57BL/6 mice (6 weeks old; weighting 19−22 g) were purchased from Beijing Vital River Laboratory Animal Technology Co., Ltd. Mice were housed in an air-conditioned room with the laboratory temperature maintained at 25 ± 2 °C, relative humidity of 50 ± 5%, 12 h of light/dark cycle, and access food and water randomly. Animal experiments were approved by the Institutional Animal Care and Use Committee of the Ocean University of China (NO.OUC-SMP-2022.08.01).
Cell culture
Caco2 cells, RAW264.7 cells, and CHO-K1 cells were purchased from the Cell Bank of the Chinese Academy Sciences (Shanghai, China). Caco2 cells were cultured in RPMI-1640 medium (Gibco, Grand Island, NY, USA) supplemented with 10% fetal bovine serum (FBS), 1% penicillin and streptomycin. RAW264.7 cells were incubated in Dulbecco’s modified eagle’s medium (DMEM) (Gibco, Grand Island, NY, USA) contained with 10% FBS. CHO-K1 cells were incubated in DynamisTM AGTTM medium (Gibco, Grand Island, NY, USA) for subculturing, then inoculated into DynamisTM AGTTM medium supplemented with a totally 30% Cell BoostTM 7a medium (Cytiva) and 3% Cell BoostTM 7b medium (Cytiva) for TFF2-Fc fusion protein producing. Caco2 cells, RAW264.7 cells, and CHO-K1 cells were cultured in a humidified incubator at 37 °C with 5% CO2, when cells were in the logarithmic phase of growth, cell experiments were started.
Construction, expression, and purification of TFF2-Fc fusion protein
To prepare TFF2-Fc fusion protein, we first constructed a stable recombinant expression vector. Briefly, the hTFF2-hIgG4-Eu (Fc) gene fragment was synthesized by Synbio Technologies (Suzhou, China). Then the gene fragment was inserted into pcDNA3.1 (+) (Ampicillin) vector after enzyme cleavage to construct the recombinant transfer vector hTFF2-hIgG4-Eu (Fc) pcDNA3.1 (+). Next, according to the manufacturer’s instructions, the recombinant transfer vector hTFF2-hIgG4-Eu (Fc) pcDNA3.1 (+) was transfected into CHO-K1 cells, and the pcDNA3.1 (+) blank vector was used as a control. After establishing a stable TFF2-Fc cell line, the fusion protein was produced in the culture medium. To purify TFF2-Fc fusion protein, the culture medium was incubated with protein A resin (Cytiva), and bound proteins were eluted with citrate buffer (pH 3.5) and neutralized by 1 M Tris‐HCl (pH 9.0). The purified fractions were analyzed by Agilent 1260 high-performance liquid chromatography (HPLC) system (Agilent, America) and sodium dodecyl sulfate (SDS)‐10% polyacrylamide gel electrophoresis, followed by staining with Coomassie brilliant blue. The resulting solution containing the fusion protein was sterilized by filtering through a 0.22 μm filter, and stored at − 80 °C until use.
Induction of ulcerative colitis and evaluations in C57BL/6 mice
C57BL/6 mice were randomly assigned into four groups (n = 7): control group, ulcerative colitis model group, SASP positive control group, and TFF2-Fc group. Ulcerative colitis mice model was induced by DSS. Briefly, mice were freely fed sterile water containing 2.5% (w/v) DSS for 8 days to establish an acute ulcerative colitis model. In the control group, mice were given drinking water every day and intrarectally administered phosphate buffer saline (PBS). The model group mice received 2.5% DSS in drinking water and intrarectally administered with PBS, SASP group mice were given 2.5% DSS and intrarectally administered SASP (50 mg/kg), and TFF2-Fc group mice were received TFF2-Fc (5 mg/kg) intrarectally once per day after fasted for 4 h during DSS treatment (Fig. 2a).
Body weight loss, stool consistency, and fecal blood loss were observed and recorded daily to evaluate the severity of ulcerative colitis. Disease activity index (DAI) was evaluated according to the scoring system shown in Table 1 (Xu et al. 2020). On day 9, all mice were scarified, and the length of colon was measured and photographed before colon tissues were collected for analysis.
Preparation of inflammatory conditioned medium (LPS-RAW264.7/CM) from RAW264.7 cells
RAW264.7 cells were seeded in 6-well plates (3 × 105 cells/well) and treated with LPS (1 μg/mL) for 24 h. After the treatment, the precipitated cells were removed after centrifugation, and the supernatant was collected as LPS-RAW264.7/CM.
Cell viability assay
Sulforhodamine B (SRB) assay was used to determine cell proliferation. In brief, Caco2 cells were planted and treated with TFF2-Fc (25−400 μg/mL) for 24 h. For the H2O2 model group, Caco2 cells were treated with a predetermined concentration of TFF2-Fc for 24 h, then incubated with H2O2 (500 μM) for 3 h. For the LPS-RAW264.7/CM model group, Caco2 cells were pretreated with TFF2-Fc for 2 h, then the culture medium was replaced with LPS-RAW264.7/CM containing TFF2-Fc and co-incubated with cells for 24 h. After incubation, the supernatant was removed, cells were fixed with 10% trichloroacetic acid for 1 h at 4℃. SRB was added and incubated for 20 min in the dark, the bound dye was dissolved with Tris buffer. Finally, the OD value was measured by a microplate reader (BioTek, Winooski, VT, USA) at 515 nm, and cell viability (%) was calculated.
3- (4,5-dimethylthiazol-2-yl) -2,5-diphenyltetrazolium bromide (MTT) assay was used to detect the effect of TFF2-Fc on the growth of RAW264.7 cells. RAW264.7 cells were incubated with different concentrations of TFF2-Fc, after treatment for 24 h, MTT (5 mg/mL) was added and incubated with cells for another 4 h. Subsequently, dimethyl sulfoxide (DMSO, 100 μL) was added and absorbance was measured at 570 nm by a microplate reader.
Cell apoptosis analysis
Generally, Caco2 cells (2 × 105 cells/well) were seeded in 6-well plates, and treated with H2O2 (500 µM) for 3 h after incubating with TFF2-Fc for 24 h. For the LPS-RAW264.7/CM model group, Caco2 cells were preincubated with TFF2-Fc for 2 h, then the original medium was removed and LPS-RAW264.7/CM containing TFF2-Fc was added. After harvesting, Caco2 cells were suspended in RPMI-1640 medium with 1% FBS and stained with Muse® Annexin V & Dead Cell Kit (Millipore, Billerica, MA, USA) for 20 min at room temperature in the dark. Muse™ cell analyzer (Microwell, Bishop, MA, USA) was used to detect the proportion (%) of apoptotic cells.
Wound healing assay
Wound healing assay was used to detect Caco2 cell migration. Caco2 cells were seeded in 96-well plates (1 × 104 cells/well). A wound was made by a 10 μL pipette tip when Caco2 cells reach 90% confluence. After treatment with TFF2-Fc in the presence or absence of H2O2 or LPS-RAW264.7/CM, the wounds were photographed at 0, 24, or 48 h using a microscope (Leica, Germany). Finally, the wound healing rate was calculated by Image J software.
Western blotting assay
Caco2 cells were seeded in 6-well plates (2 × 105 cells/well) and treated with TFF2-Fc (0−200 μg/mL) followed by H2O2 or LPS-RAW264.7/CM treatment. RAW264.7 cells were seeded and incubated with TFF2-Fc before LPS treatment. Subsequently, the cells were collected and lysed. The cell’s lysates were separated by electrophoresis on SDS–polyacrylamide gel electrophoresis (PAGE) (6−10%), and transferred to a nitrocellulose filter membrane (Millipore, Billerica, MA, USA). Furthermore, the nitrocellulose membrane was blocked with 5% skim milk, and the primary antibody was added. After overnight incubation, the nitrocellulose membrane was incubated with HRP-secondary antibody for 1 h at 25 °C. Finally, the image was detected by Tanon 5200 (Tanon, Beijing, China).
Enzyme-linked immunosorbent (ELISA) assay
ELISA assay was used to detect the level of inflammation cytokines in RAW264.7 cells. Briefly, RAW264.7 cells were pretreated with TFF2-Fc (0−200 µg/mL) for 2 h before being incubated with LPS (100 ng/mL) for 24 h. After incubation, the supernatant was collected and centrifuged. According to the manufacturer’s instructions (Dakewe, Beijing, China), the content of TNF-α, interleukin-6 (IL-6) was measured.
Nitrite oxide (NO) and glycolysis analysis
After treatment, the supernatant of RAW264.7 cells was collected, and the concentration of NO was determined according to NO kit (Beyotime, Shanghai, China) instructions. Glucose and lactate levels were also determined according to the manufacturers’ instructions.
Determination of reactive oxygen species (ROS)
RAW264.7 cells were pretreated with TFF2-Fc (100, 200 µg/mL) for 2 h, LPS (100 ng/mL) was added for another 24 h. Then cells were stained with DCFH-DA (10 µM, Beyotime, Shanghai, China) according to the manufacturer’s instructions, and the fluorescence signals were analyzed through CytoFLEX (Beckman Coulter, Suzhou, China).
Flow cytometry for macrophage subset analysis
After treatment, according to the manufacturer’s instructions (BD Biosciences, USA), RAW264.7 cells were incubated with monoclonal antibodies against CD86 (M1 marker), CD206 (M2 marker), and the corresponding fluorescent markers. Then RAW264.7 cells were further analyzed using a flow cytometer.
Statistical analysis
The data shown in this study were represented as the mean ± SD. Comparisons between the groups were assessed by one-way analysis of variance (ANOVA), and a multiple comparison test was performed using the post hoc Bonferroni correction. P < 0.05 was defined as statistically significant.
Results
Generation of TFF2-Fc fusion protein
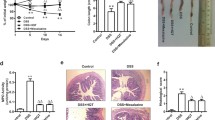
TFF2 was fused with the Fc fragment of human IgG4, constructed in pcDNA3.1 (+), and TFF2-Fc was expressed in the stable CHO-K1 cell line (Fig. 1a). TFF2-Fc fusion protein was purified from the culture supernatant using a Protein A column with a yield up to 10 mg/mL. The purity and size of TFF2-Fc protein were evaluated by running HPLC and SDS-PAGE gel under reduced and non-reduced conditions. As shown in Fig. 1b, HPLC results showed that the purity of fusion protein was 99.26%. As shown in Fig. 1c, the SDS-PAGE data showed that the size of purified TFF2-Fc fusion protein was about 50 kDa under reduced conditions (Line 1−4), and the size of TFF2-Fc was about 100 kDa under non-reduced conditions (Line 5−8). These results showed that TFF2-Fc existed as a dimer with a molecular weight of 100 kDa.
Construction and identification of recombinant protein TFF2-Fc. a Construction map of hTFF2-hIgG4-Eu (Fc) pcDNA3.1 (+). b HPLC assay was used to determine the purity of TFF2-Fc. Peak 1: Impurity peak A. Peak 2: TFF2-Fc peak. Peak 3: Impurity peak B. c SDS-PAGE of purified TFF2-Fc. Lane M: Molecular weight standard. Lane 1−4: SDS-PAGE was conducted under reducing condition, TFF2-Fc (5, 10 µg) was mixed with loading buffer containing mercaptothion and boiled at 100 ℃ for 10 min. Lane 5−8: SDS-PAGE was conducted under non-reducing condition, TFF2-Fc (5, 10 µg) was mixed with non-reducing loading buffer and boiled at 100 ℃ for 10 min. Then the SDS-PAGE gel was stained with Coomassie Brilliant Blue R-250
TFF2-Fc attenuates DSS-induced ulcerative colitis in mice
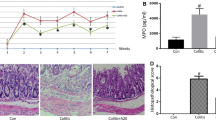
DAI score is a clinical parameter reflecting the severity of ulcerative colitis. DAI score was compared in a different group, as shown in Fig. 2b, the DAI score was obviously increased in the DSS model group from day 3 compared with the control group. However, the DAI score was significantly reduced in the SASP-treated group from day 4. And this elevation of DAI score in the DSS model group was relieved by the treatment of TFF2-Fc from day 4 (Fig. 2b). On day 3, TFF2-Fc treatment significantly attenuated the body weight loss during experimental colitis disease progression in mice compared with the model group, and the SASP group showed a similar tendency (Fig. 2c). Consistent with the weight loss, DSS-induced hematochezia was recovered in the TFF2-Fc treatment group from day 4, the symptoms of hematochezia were also relieved in SASP group (Fig. 2d). As an indicator of the indirect reflection of colonic inflammation, the length of the colon was also measured. As shown in Fig. 2e and f, after 8 days treatment, TFF2-Fc significantly inhibited DSS-induced colon shortening, and the shortening of the colon was also improved by SASP treatment. These results indicated that TFF2-Fc could mitigate the symptoms of DSS-induced ulcerative colitis in mice.
TFF2-Fc treatment decreases the susceptibility of DSS-induced ulcerative colitis in C57BL/6 mice. a Ulcerative colitis mice induced by 2.5% DSS and treated with SASP (50 mg/kg) or TFF2-Fc (5 mg/kg) for 8 days. b DAI score. Mice were given 2.5% DSS or water for 8 day, SASP (50 mg/kg) or TFF2-Fc (5 mg/kg) was intrarectally administrated daily and DAI score were calculated. c Changes in body weight were recorded daily. d Bloody stools during experimental colitis. e Representative images of colons from each group of mice. f The lengths of colons from each group of mice were measured. g Representative images of colonic tissue sections with hematoxylin & eosin (H&E) staining, scale bar, 1000 μm (upper panel), 100 μm (lower panel). The panel in the lower, in which the colonic epithelium was marked with a black arrow and goblet cells were circled in red, is magnified from the position indicated by the blue arrow in the upper panel. #P < 0.05, ##P < 0.01 versus the control group, *P < 0.05, **P < 0.01 versus the model group (color figure online)
Then, we analyzed mucosa and goblet cells in colonic tissues to investigate the histopathological changes after TFF2-Fc treatment. Based on the results of H&E staining (Fig. 2g), we observed well-preserved mucosa (black arrow) and normal goblet cells (circled in red) in the colon of control mice. However, the DSS-treated mice colon exhibited significant inflammatory symptoms, including lymphocyte infiltration (white arrow), goblet cell depletion (circled in red), and inconspicuous epithelial layer destruction (black arrow). Positive control SASP group showed only moderate epithelial damage and goblet cell depletion. Interestingly, TFF2-Fc treatment reversed the lesions in the epithelial layer (black arrow) and goblet cells (circled in red) (Fig. 2g), indicating that TFF2-Fc was able to significantly alleviate the severe histopathological damage caused by DSS treatment. These results suggested that TFF2-Fc provided a protective effect against DSS-induced mucosal injury in mice.
TFF2-Fc ameliorates Caco2 cells damage induced by H2O2 or LPS-RAW264.7/CM
We then investigated the effect of TFF2-Fc on the proliferation of intestinal epithelial cells. As shown in Fig. 3a, TFF2-Fc (50−400 μg/mL) slightly promoted the proliferation of Caco2 cells. As shown in Fig. 3b, Caco2 cells viability was reduced to 70.66% after treated with H2O2 (500 μM) compared with a control group. TFF2-Fc could increase the cells viability to 89.16, 97.19, and 91.60% at a concentration of 50, 100, and 200 μg/mL, respectively. LPS could stimulate RAW264.7 cells to secrete many inflammatory factors (Jakkawanpitak et al. 2020), which could be sensed by intestinal epithelial cells, and cause inflammatory damage. LPS-RAW264.7/CM was widely used in the model of cell inflammatory injury. Next, we examined the effect of TFF2-Fc on LPS-RAW264.7/CM-induced Caco2 cells damage. As shown in Fig. 3c, Caco2 cells viability was reduced to 75.64% after being treated with LPS-RAW264.7/CM, and the TFF2-Fc pre-treatment alleviated this cell damage induced by LPS-RAW264.7/CM at a concentration of 100 and 200 μg/mL, with the cells viability increased to 87.05, 91.05%, respectively. These results indicated that TFF2-Fc pretreatment exhibited a significant protective effect on intestinal epithelial cells in oxidative and inflammatory damage.
TFF2-Fc ameliorates the cell damage induced by H2O2 or LPS-RAW264.7/CM in Caco2 cells. a The effect of TFF2-Fc on the Caco2 cells viability. Caco2 cells were seeded in 96-well plates and treated with different concentrations of TFF2-Fc for 24 h. b Caco2 cells were incubated with TFF2-Fc (0−400 µg/mL) for 24 h, then treated with H2O2 (500 μM) for 3 h. c Caco2 cells were pre-incubated with TFF2-Fc (0−400 µg/mL) for 2 h, then the culture medium was replaced with LPS-RAW264.7/CM containing TFF2-Fc for 24 h. After incubation, cell viability was tested by the SRB method. Values are expressed as mean ± SD of three independent experiments. *P < 0.05, **P < 0.01, versus control group, #P < 0.05, ##P < 0.01, versus H2O2 or LPS-RAW264.7/CM model group
TFF2-Fc ameliorates H2O2 or LPS-RAW264.7/CM-induced apoptosis in Caco2 cells
We further tested the effect of TFF2-Fc on Caco2 cells apoptosis induced by H2O2 or LPS-RAW264.7/CM. As shown in Fig. 4a, H2O2 (500 µM) treatment alone could increase the percentage of Caco2 apoptotic cells to 36.82% and decrease the percentage of living cells to 57.21%. However, TFF2-Fc treatment reduced the proportion of apoptotic cells to 22.30, 19.93, and 22.32%, and increased the proportion of living cells to 71.06, 73.60, and 70.31% at concentrations of 50, 100, and 200 μg/mL. Consistently, as shown in Fig. 4b, the proportion of apoptotic cells significantly increased to 25.82%, and the percentage of live cells decreased to 72.53% after LPS-RAW264.7/CM treatment alone. TFF2-Fc (50−200 μg/mL) pretreatment could decrease the proportion of apoptotic cells to 25.47, 11.67, and 10.41%, and increase the proportion of living cells to 73.32, 86.80, and 88.52%. The above results showed that TFF2-Fc could alleviate Caco2 cells apoptosis induced by oxidative or inflammatory damage.
TFF2-Fc ameliorates H2O2 or LPS-RAW264.7/CM induced apoptosis in Caco2 cells. Caco2 cells were seeded in 6-well plates (2 × 105 cells/well), and treated with TFF2-Fc (50−200 μg/mL) in the presence or absence of H2O2 a or LPS-RAW264.7/CM b, then the ratio of apoptosis Caco2 cells was measured with a Muse™ cell analyzer. The bar graph depicts the percentage of apoptotic and living Caco2 cells induced by TFF2-Fc in the absence or presence of H2O2 or LPS-RAW264.7/CM. Values are expressed as the mean ± SD of three independent experiments. *P < 0.05, **P < 0.01, versus control group, #P < 0.05, ##P < 0.01, versus H2O2 or LPS-RAW264.7/CM model group
TFF2-Fc promotes the migration of Caco2 cells
Intestinal epithelial cells migration is critical for the remission process of ulcerative colitis. The effect of TFF2-Fc on Caco2 cells migration was detected by wound healing assay. The results showed that, compared with a control group (39.99%), the wound healing rates of Caco2 cells treated with TFF2-Fc (100−400 μg/mL) were 51.03, 64.26, and 51.96%, respectively (Fig. 5a and d). To further verify whether TFF2-Fc promoted the migration of damaged intestinal epithelial cells, H2O2 or LPS-RAW264.7/CM was used to prepare an oxidative or inflammatory injury model. As shown in Fig. 5b and e, compared with the healing rate of the control group (22.48%), the Caco2 cells migration rate of the H2O2 model group was significantly reduced to 8.7%. TFF2-Fc pretreatment could upregulate the Caco2 cells migration rate inhibited by H2O2, the migration rate increased to 12.41, 14.34, and 16.79% at a concentration of 100, 200, and 400 μg/mL, respectively. Similarly, Caco2 cells migration rate was significantly reduced to 4.04% after LPS-RAW264.7/CM treatment (Fig. 5c and f), and TFF2-Fc pretreatment could effectively alleviate the suppression of cell migration induced by LPS-RAW264.7/CM, the migration rate increased to 11.99, 12.24, and 20.87% at a concentration of 100, 200, and 400 μg/mL. These results suggested that TFF2-Fc could promote Caco2 cells migration after oxidative injury and inflammatory injury.
The effects of TFF2-Fc on the migratory ability of Caco2 cells. a Caco2 cells were wounded with 10 μL pipette tip when the cells reach 90% confluence, then the cells were allowed to migrate for 48 h in the absence or presence of TFF2-Fc. The cells were pretreated with TFF2-Fc for 2 h, then treated with H2O2 b or LPS-RAW264.7/CM c for 24 h. Subsequently, the wound healing area was calculated by Image J software. d, e, and f Histogram shows the wound healing area after different treatments. Values are expressed as the mean ± SD of three independent experiments. *P < 0.05, **P < 0.01, versus control group. #P < 0.05, ##P < 0.01, versus H2O2 or LPS-RAW264.7/CM model group
TFF2-Fc reverses the expression of tight junction proteins down-regulated by H2O2 or LPS-RAW264.7/CM in Caco2 cells
The disruption of tight junction proteins, including Occludin and ZO-1, will lead to impaired mucosal, and promote the occurrence and development of ulcerative colitis. Therefore, we further examined the expression of Occludin and ZO-1 affected by TFF2-Fc in Caco2 cells. In the H2O2 model group, as shown in Fig. 6a and b, we found that H2O2 treatment decreased the level of Occludin and ZO-1, TFF2-Fc pretreatment could increase the expression of tight junction proteins. To further confirm this result, we treated Caco2 cells with LPS-RAW264.7/CM. In Fig. 6c and d, similarly, the expression of Occludin and ZO-1 were significantly reduced after LPS-RAW264.7/CM treatment, while the co-incubation of TFF2-Fc and LPS-RAW264.7/CM significantly up-regulated the expression of tight junction proteins. The result suggested that TFF2-Fc protected intestinal epithelial cells from injury at least partially via reversing the reduction of tight junction proteins level.
The effect of TFF2-Fc on the expression of Occludin and ZO-1 in Caco2 cells. a, b Caco2 cells were treated with TFF2-Fc (100 − 200 µg/mL) for 24 h or 2 h, then incubated with or without H2O2 (500 or 60 µM) for 3 h or 24 h, respectively. c, d Caco2 cells were pretreated with TFF2-Fc (100−200 µg/mL) for 2 h and co-incubated with or without LPS-RAW264.7/CM for 24 h. The level of tight junction proteins was detected by western blotting assay. Values are expressed as the mean ± SD of three independent experiments. *P < 0.05, **P < 0.01, versus control group, #P < 0.05, ##P < 0.01, versus H2O2 or LPS-RAW264.7/CM model group
TFF2-Fc promotes P-ERK activation in Caco2 cells
Studies have shown that TFF2 could stimulate ERK phosphorylation by binding to the chemokine receptor (CXCR4), thereby promoting cell proliferation, migration, and survival (Iyengar and Jangra 2019). To further verify whether TFF2-Fc also repair intestinal epithelial cells by affecting CXCR4/ERK pathway, we detected the activation of CXCR4 downstream molecule P-ERK. H2O2 was used to damage Caco2 cells and inhibit P-ERK activation. As shown in Fig. 7a, H2O2 (500 μM) treatment significantly inhibited P-ERK activation, while TFF2-Fc promoted the level of P-ERK inhibited by H2O2, indicating that TFF2-Fc could play a repair role by affecting the activation of P-ERK. This ERK activation promoted by TFF2-Fc was inhibited by ERK inhibitor U0126 (Fig. 7b), consequently, the repair effect of TFF2-Fc on the damaged Caco2 cells was also inhibited by U0126 (Fig. 7c), which further confirming that TFF2-Fc could repair intestinal epithelial cells via stimulating CXCR4/ERK signal pathway.
The effect of TFF2-Fc on the level of P-ERK in Caco2 cells. Caco2 cells were treated with different concentrations of TFF2-Fc for 24 h or 2 h, then incubated with or without H2O2 a or LPS-RAW264.7/CM d for 3 h or 24 h. The activation of P-ERK was detected by western blotting assay. b Caco2 cells were treated with U0126 (5 µM) for 2 h, then treated with TFF2-Fc (100 µg/mL) for 22 h and then treated with H2O2 (500 µM) for 3 h, cells were collected and the level of P-ERK was detected. Histogram shows the relative abundance of P-ERK to the control group. c SRB assay was used to detect Caco2 cells viability. Caco2 cells were treated with U0126 (5 µM) for 2 h, then treated with TFF2-Fc (100 µg/mL) for 22 h and then treated with H2O2 (500 µM) for 3 h. Values are expressed as the mean ± SD of three independent experiments. *P < 0.05, **P < 0.01, versus control group, #P < 0.05, ##P < 0.01, versus H2O2 model group
Interestingly, different to the H2O2 treatment, P-ERK in Caco2 cells treated with LPS-RAW264.7/CM was activated (Fig. 7d). This is possibly due to the presence of many inflammatory factors in LPS-RAW264.7/CM, including TNF-α, and IL-6, which might lead to the protective activation of P-ERK, the binding of TFF2-Fc to CXCR4 further activated P-ERK. To some extent, this is similar to TFF3 could up-regulate LPS-activated P-AKT in gastric epithelial cells (Huang et al. 2020). In general, these results suggested that TFF2-Fc could promote the proliferation and migration of Caco2 cells by activating P-ERK.
TFF2-Fc inhibits LPS-stimulated inflammation in RAW264.7 cells
To investigate the anti-inflammatory effect of TFF2-Fc, we examined the level of inflammatory cytokines in RAW264.7 cells after being treated with LPS. As shown in Fig. 8a, TFF2-Fc (25−400 μg/mL) alone did not affect the viability of RAW264.7 cells. The level of TNF-α (Fig. 8b), IL-6 (Fig. 8c), and NO (Fig. 8d) was upregulated significantly after LPS stimulation, while TFF2-Fc decreased the secretion of LPS-induced inflammatory cytokines. The continuous production of inflammatory factors will cause the excessive expression of ROS and further lead to oxidative damage. As shown in Fig. 8e and f, TFF2-Fc alone did not change the level of ROS in RAW264.7 cells, and the ROS level was significantly increased after LPS stimulation in RAW264.7 cells, which was about 7 times that of the control group. However, TFF2-Fc could effectively reduce LPS-induced ROS elevation at the concentration of 100 and 200 μg/mL. These results indicated that TFF2-Fc could reduce cell inflammation and subsequently reduce oxidative damage.
TFF2-Fc ameliorates LPS-induced inflammation in RAW264.7 cells. a The effect of TFF2-Fc on the RAW264.7 cells viability. RAW264.7 cells were treated with TFF2-Fc (0−800 µg/mL) for 24 h after adherence, and cells viability was tested using the MTT method. The level of TNF-α b, IL-6 c and NO d of RAW264.7 cells was detected. RAW264.7 cells were plated in 6-well plates (3 × 105 cells/well) and pre-treated with TFF2-Fc (0−200 µg/mL) for 2 h, LPS (100 ng/mL) was added in RAW264.7 cells for 24 h. Then the level of inflammatory factors in the supernatant was detected according to the manufacturer’s instructions. e After treatment with or without TFF2-Fc and LPS, RAW264.7 cells were collected and stained with DCFH-DA (10 µM) for 20 min, the fluorescence signals were detected by CytoFLEX. f Histogram shows the mean fluorescence of ROS with or without TFF2-Fc or LPS in RAW264.7 cells. g RAW264.7 cells were collected and the level of P-ERK was detected by western blotting. h Histogram shows the relative abundance of P-ERK to the control group. *P < 0.05, **P < 0.01, versus control group, #P < 0.05, ##P < 0.01, versus LPS model group
It has been shown that inhibiting the activation of P-ERK promoted by LPS could reduce the inflammatory response in RAW264.7 cells (Zhou et al. 2019). Therefore, we investigated the effect of TFF2-Fc on P-ERK level in RAW264.7 cells. As shown in Fig. 8g and h, P-ERK was significantly activated after LPS stimulation compared with the control group, however, TFF2-Fc pretreatment did not inhibit the activation of P-ERK promoted by LPS, which was possibly related to the expression of CXCR4 receptor on RAW264.7 cells, and TFF2-Fc could bind to CXCR4 and further activate ERK signaling pathway. These results suggested that TFF2-Fc could inhibit LPS-induced inflammation in RAW264.7 cells through other signaling pathways, while not CXCR4/ERK pathway.
TFF2-Fc alleviates LPS-induced M1 polarization in RAW264.7 cells by reducing glycolysis
The M1 phenotype of macrophages could secrete pro-inflammatory factors and play a pro-inflammatory function, and M1 phenotype of RAW264.7 cells induced by LPS is a classic model of inflammation. It has been reported that pre-incubation of microglia with TFF3 leads to a reduction of the M1 phenotype (Arnold et al. 2016), but the effect of TFF2 on macrophage polarization remains unclear. Next, we examined the effect of TFF2-Fc on LPS-induced M1 polarization in RAW264.7 cells. As shown in Fig. 9a and b, the proportion of CD86+ cells significantly increased after LPS treatment, while the proportion of CD206+ cells did not change, indicating that LPS could cause normal RAW264.7 cells to differentiate into M1 phenotype cells. However, the CD86+ cell populations were decreased remarkably when treated with TFF2-Fc (100 and 200 μg/mL), while not affecting the proportion of CD206+ cells. The result suggests that TFF2-Fc could inhibit the polarization of macrophages to M1 phenotype. The energy required for macrophage polarization mainly comes from glycolysis (Wu et al. 2021), and whether TFF2 plays an anti-inflammatory role by inhibiting glycolysis has not been reported. Therefore, we further examined the effect of TFF2-Fc on glucose consumption and lactate secretion after LPS-induced polarization of RAW264.7 cells. As shown in Fig. 9d and e, glucose consumption and lactate secretion were increased significantly after being treated with LPS. TFF2-Fc pretreatment could significantly inhibit LPS-induced glucose consumption and lactate secretion, and thus inhibited macrophage polarization induced by LPS. This result further demonstrated that TFF2-Fc could attenuate the inflammatory response by inhibiting the M1 phenotype and glycolysis of macrophages.
TFF2-Fc could inhibit LPS-induced M1 polarization of RAW264.7 cells by reducing glycolysis. a RAW264.7 cells were seeded in 6-well plates and treated with TFF2-Fc for 2 h before exposed LPS (100 ng/mL) for 24 h. Then cells were collected and the proportion of CD86+ cells was detected by flow cytometry according to the manufacturer’s instructions. Histogram shows the proportion of (b) CD86+ cells and (c) CD206+ cells in total cells. Glucose consumption (d) and lactate production (e) was measured in the supernatant by kit. Values are expressed as the mean ± SD of three independent experiments. **P < 0.01, ***P < 0.001, versus control group, #P < 0.05, ###P < 0.001, versus LPS model group
Discussions
In recent 30 years, the incidence of ulcerative colitis has been increasing, ulcerative colitis has gradually become a common disease in gastroenterology (Mak et al. 2020). Current drugs mainly treat ulcerative colitis by reducing inflammation, but these traditional anti-inflammatory chemical drugs might cause nausea, vomiting, and other gastrointestinal side effects. The TNF-α inhibitor infliximab has a good therapeutic effect in one-third of patients, however, it also increased the risk of serious infections and malignancy (Na and Moon 2019). In addition to reducing the inflammatory response, repairing the damaged mucosa is a promising effective strategy for the treatment of ulcerative colitis. For example, it has been reported that IL-33 could alleviate colitis by repairing the damaged gastrointestinal mucosa (Lopetuso et al. 2018), and polysaccharide from Scutellaria baicalensis Georgi has also been shown to relieve ulcerative colitis by repairing damaged intestinal epithelial barriers (Cui et al. 2021).
Recent studies indicated that TFF2 could promote lung epithelial repair and reduce the symptom of COVID-19 (Fu et al. 2020). Moreover, most interestingly, TFF2 promoted injured gastrointestinal mucosa repair and reduced inflammatory response in rats (Tran et al. 1999). TFF2 also played a crucial role in repairing the DSS-damaged gastrointestinal mucosa in mice, TFF2 promoting the migration of neighboring cells, and maintaining the epithelial integrity of gastrointestinal mucosa (Aihara et al. 2017), these evidences suggest that TFF2 might have potential significance in the treatment of ulcerative colitis. However, the short half-life of TFF2 (48 min in mice) limited its further development and application (Kjellev et al. 2007). It has been studied that the half-life of Apelin-13 was 8 min in blood, while the half-life of Fc-Apelin-13 fusion protein was 33 h (Wang et al. 2018). The half-life of ELA-21 was 13 min, while the Fc-ELA-21 (44 h) fusion protein had a half-life approximately 203 times that of ELA-21 (Xi et al. 2019). Therefore, we introduced the Fc fragment and successfully prepared the recombinant protein TFF2-Fc, which prolong the half-life of the protein, and also simplify the separation, with a yield of 10 mg/mL and a purity of 99.26%. The result indicated that we established an efficient method to prepare the fusion protein TFF2-Fc for the first time, which will also greatly prolong the serum half-life of TFF2, and the exact half-life of TFF2-Fc will be further determined in our subsequent pharmacokinetic experiments.
Then we further investigated whether TFF2-Fc, like TFF2, could repair gastrointestinal mucosa and suppress inflammation. Consistent with TFF2 (Tran et al. 1999), we found that TFF2-Fc could also reduce DAI score, improve body weight, blood stool, and colon length in C57BL/6 mice (Fig. 2). In the colon, goblet cells could form a mucosal barrier to protect epithelial cells by synthesizing and secreting mucins, which is critical for maintaining normal colon function. H&E staining confirmed that TFF2-Fc effectively ameliorated goblet cell depletion and inflammatory cell infiltration induced by DSS in the intestinal tract.
Rapid proliferation and renewal of gastrointestinal epithelial cells is a key step to repair injured intestinal mucosal (Hoffmann 2005). Gastrointestinal epithelial cell activities, such as survival, migration, and proliferation could be regulated via stimulating CXCR4/ERK signaling pathway. It has been reported that WINT16B (a secreted protein that could activate both canonical and non-canonical WNT signaling pathways) could enhance the proliferation and self-renewal of corneal epithelial cells, and accelerate corneal epithelial wound healing through CXCR4/ERK signaling cascade (Zhao et al. 2022). Stromal cell-derived factor/chemokines-12 could regulate the maturation and restoration of the intestinal epithelial cells via CXCR4/ERK signaling pathway, and restore the integrity of the mucosal barrier in vivo (Zimmerman et al. 2011). Previous studies have revealed that TFF2 could drive calcium mobilization and recovery of injured gastric mucosa by stimulating the CXCR4 receptor (Engevik et al. 2019). Our study found that TFF2-Fc could also stimulate CXCR4/ERK signaling pathway, and subsequently promote the viability and migration of intestinal epithelial cells.
In addition to cell viability and migration, the integrity of the epithelial cells is also important for the treatment of ulcerative colitis. In intestinal epithelial cells, tight junction proteins are essential for maintaining integrity, P-ERK also plays an indispensable role in regulating the expression of tight junction proteins (Basuroy et al. 2006). It has been shown that VSL#3 probiotics could protect the epithelial barrier by activating the ERK signaling pathway, increasing the expression of tight junction proteins in vivo and in vitro, and alleviating DSS-induced acute colitis in rats (Dai et al. 2012). Considering TFF2 could also affect tight junction proteins and maintain the epithelial barrier via the activation of CXCR4/ERK, and our present results regarding the P-ERK activation and the tight junction proteins elevation, we confirmed that TFF2-Fc could protect tight junction proteins from oxidative damage and inflammatory damage by stimulating the CXCR4/ERK pathway, and further indicating the importance of CXCR4/ERK pathway in the prevent and therapy of ulcerative colitis. In fact, many macromolecules, including human β-defensin-3, stromal cell-derived factor, have already been reported to activate the CXCR4/ERK pathway, and showed potential benefit in the treatment of ulcerative colitis (Chen et al. 2019; Liu et al. 2014). Thus, it is also a valid strategy to find ulcerative colitis drugs from the compounds which activate the CXCR4/ERK pathway. In addition, whether the repair effect of TFF2-Fc could be applied to other mucosal repair-related diseases, such as oral ulcers, needs further investigation.
Various pro-inflammatory factors produced by M1 macrophages are key substances that aggravate ulcerative colitis symptoms (Chu et al. 2019). It has been reported that TFF3 could inhibit the polarization of microglia to the M1 phenotype and alleviate neuroinflammation. However, the effect of TFF2 on macrophage polarization has not been reported. For the first time, our work showed that TFF2-Fc could inhibit the polarization of macrophages to the M1 phenotype, and thereby reducing the production of inflammatory factors and inhibit the inflammatory response. Moreover, we found TFF2-Fc could inhibit glycolysis by inhibiting glucose consumption and lactate production in macrophages, which is the energy source for macrophage polarization to the M1 phenotype. Whether the inhibiting inflammatory response activity of TFF2-Fc contributes to the treatment of other inflammatory diseases, such as COVID-19-related pneumonia, has to be yet investigated. In addition to the alleviation of inflammation of ulcerative colitis, for the first time we have demonstrated that TFF2-Fc significantly promoted the repair of the gastrointestinal mucosa, which was totally different from the current clinical therapeutic drugs. The dual role of TFF2-Fc demonstrated that TFF2-Fc could be used to clinically strengthen traditional anti-inflammatory drugs to achieve better outcomes and/or to be used as a single non-toxic bioactive substance to promote the regeneration of gastrointestinal mucosa.
Conclusions
In summary, for the first time, our present work has established an efficient method to prepare TFF2-Fc fusion protein, with a yield of 10 mg/mL and a purity of 99.26%. TFF2-Fc could significantly improve the symptoms of ulcerative colitis mice. In intestinal epithelial cells, TFF2-Fc inhibited cellular injury and apoptosis, promoted cells migration, and increased the expression of tight junction proteins by stimulating CXCR4/ERK signaling pathway. Moreover, TFF2-Fc could reduce the inflammatory response of macrophages via inhibiting glucose consumption and lactate production. Our present work demonstrated the therapeutic effect of TFF2-Fc in ulcerative colitis attributed to both inflammatory inhibition and gastrointestinal mucosa regeneration, this dual role of TFF2-Fc suggested that it could be served as a novel bioactive substance for alternative treatment for ulcerative colitis.
Data availability
Enquiries about data availability should be directed to the authors.
References
Agagunduz D, Gencer Bingol F, Celik E, Cemali O, Ozenir C, Ozogul F, Capasso R (2022a) Recent developments in the probiotics as live biotherapeutic products (LBPs) as modulators of gut brain axis related neurological conditions. J Transl Med 20:460. https://doi.org/10.1186/s12967-022-03609-y
Agagunduz D, Kocaadam-Bozkurt B, Bozkurt O, Sharma H et al (2022b) Microbiota alteration and modulation in Alzheimer’s disease by gerobiotics: the gut-health axis for a good mind. Biomed Pharmacother 153:113430. https://doi.org/10.1016/j.biopha.2022.113430
Aihara E, Engevik KA, Montrose MH (2017) Trefoil factor peptides and gastrointestinal function. Annu Rev Physiol 79:357–380. https://doi.org/10.1146/annurev-physiol-021115-105447
Arnold P, Rickert U, Helmers AK et al (2016) Trefoil factor 3 shows anti-inflammatory effects on activated microglia. Cell Tissue Res 365(1):3–11. https://doi.org/10.1007/s00441-016-2370-5
Basuroy S, Seth A, Elias B, Naren AP, Rao R (2006) MAPK interacts with occludin and mediates EGF-induced prevention of tight junction disruption by hydrogen peroxide. Biochem J 393(Pt 1):69–77. https://doi.org/10.1042/BJ20050959
Boal Carvalho P, Cotter J (2017) Mucosal healing in ulcerative colitis: a comprehensive review. Drugs 77(2):159–173. https://doi.org/10.1007/s40265-016-0676-y
Chen L, Lv Z, Gao Z et al (2019) Human beta-defensin-3 reduces excessive autophagy in intestinal epithelial cells and in experimental necrotizing enterocolitis. Sci Rep 9(1):19890. https://doi.org/10.1038/s41598-019-56535-3
Chu Q, Yu X, Jia R et al (2019) Flavonoids from Apios americana Medikus leaves protect RAW264.7 cells against inflammation via inhibition of MAPKs, Akt-mTOR pathways, and Nfr2 activation. Oxid Med Cell Longev 2019:1563024. https://doi.org/10.1155/2019/1563024
Cui L, Guan X, Ding W et al (2021) Scutellaria baicalensis Georgi polysaccharide ameliorates DSS-induced ulcerative colitis by improving intestinal barrier function and modulating gut microbiota. Int J Biol Macromol 166:1035–1045. https://doi.org/10.1016/j.ijbiomac.2020.10.259
da Silva VC, de Araujo AA, de Souza Araujo DF et al (2018) Intestinal anti-inflammatory activity of the aqueous extract from Ipomoea asarifolia in DNBS-induced colitis in rats. Int J Mol Sci. https://doi.org/10.3390/ijms19124016
Dai C, Zhao DH, Jiang M (2012) VSL#3 probiotics regulate the intestinal epithelial barrier in vivo and in vitro via the p38 and ERK signaling pathways. Int J Mol Med 29(2):202–208. https://doi.org/10.3892/ijmm.2011.839
Engevik KA, Hanyu H, Matthis AL et al (2019) Trefoil factor 2 activation of CXCR4 requires calcium mobilization to drive epithelial repair in gastric organoids. J Physiol 597(10):2673–2690. https://doi.org/10.1113/JP277259
Feuerstein JD, Moss AC, Farraye FA (2019) Ulcerative colitis. Mayo Clin Proc 94(7):1357–1373. https://doi.org/10.1016/j.mayocp.2019.01.018
Fu W, Liu Y, Xia L et al (2020) A clinical pilot study on the safety and efficacy of aerosol inhalation treatment of IFN-kappa plus TFF2 in patients with moderate COVID-19. EClinicalMedicine 25:100478. https://doi.org/10.1016/j.eclinm.2020.100478
Hoffmann W (2005) Trefoil factors TFF (trefoil factor family) peptide-triggered signals promoting mucosal restitution. Cell Mol Life Sci 62(24):2932–2938. https://doi.org/10.1007/s00018-005-5481-9
Huang Y, Wang MM, Yang ZZ et al (2020) Pretreatment with intestinal trefoil factor alleviates stress-induced gastric mucosal damage via Akt signaling. World J Gastroenterol 26(48):7619–7632. https://doi.org/10.3748/wjg.v26.i48.7619
Iyengar Y, Jangra R (2019) Trefoil factor 2-C-X-C chemokine receptor 4-mediated calcium signalling is implicated in gastric epithelial restitution. J Physiol 597(18):4685–4687. https://doi.org/10.1113/JP278602
Jahan R, Shah A, Kisling SG et al (2020) Odyssey of trefoil factors in cancer: diagnostic and therapeutic implications. Biochim Biophys Acta Rev Cancer 1873(2):188362. https://doi.org/10.1016/j.bbcan.2020.188362
Jakkawanpitak C, Hutadilok-Towatana N, Sermwittayawong D (2020) Fungal-like particles and macrophage-conditioned medium are inflammatory elicitors for 3T3-L1 adipocytes. Sci Rep 10(1):9437. https://doi.org/10.1038/s41598-020-66283-4
Kjellev S, Vestergaard EM, Nexo E et al (2007) Pharmacokinetics of trefoil peptides and their stability in gastrointestinal contents. Peptides 28(6):1197–1206. https://doi.org/10.1016/j.peptides.2007.03.016
Liu X, Zuo D, Fan H et al (2014) Over-expression of CXCR4 on mesenchymal stem cells protect against experimental colitis via immunomodulatory functions in impaired tissue. J Mol Histol 45(2):181–193. https://doi.org/10.1007/s10735-013-9541-4
Liu C, Yan X, Zhang Y et al (2022) Oral administration of turmeric-derived exosome-like nanovesicles with anti-inflammatory and pro-resolving bioactions for murine colitis therapy. J Nanobiotechnol 20(1):206. https://doi.org/10.1186/s12951-022-01421-w
Lopetuso LR, De Salvo C, Pastorelli L et al (2018) IL-33 promotes recovery from acute colitis by inducing miR-320 to stimulate epithelial restitution and repair. Proc Natl Acad Sci USA 115(40):E9362–E9370. https://doi.org/10.1073/pnas.1803613115
Mak WY, Zhao M, Ng SC, Burisch J (2020) The epidemiology of inflammatory bowel disease: east meets west. J Gastroenterol Hepatol 35(3):380–389. https://doi.org/10.1111/jgh.14872
Na SY, Moon W (2019) Perspectives on current and novel treatments for inflammatory bowel disease. Gut Liver 13(6):604–616. https://doi.org/10.5009/gnl19019
Neurath MF (2014) Cytokines in inflammatory bowel disease. Nat Rev Immunol 14(5):329–342. https://doi.org/10.1038/nri3661
Oertel M, Graness A, Thim L et al (2001) Trefoil factor family-peptides promote migration of human bronchial epithelial cells: synergistic effect with epidermal growth factor. Am J Respir Cell Mol Biol 25(4):418–424. https://doi.org/10.1165/ajrcmb.25.4.4429
Pagano E, Romano B, Iannotti FA et al (2019) The non-euphoric phytocannabinoid cannabidivarin counteracts intestinal inflammation in mice and cytokine expression in biopsies from UC pediatric patients. Pharmacol Res 149:104464. https://doi.org/10.1016/j.phrs.2019.104464
Pagano E, Iannotti FA, Piscitelli F et al (2021) Efficacy of combined therapy with fish oil and phytocannabinoids in murine intestinal inflammation. Phytother Res 35(1):517–529. https://doi.org/10.1002/ptr.6831
Pan Q, Lin S, Li Y et al (2021) A novel GLP-1 and FGF21 dual agonist has therapeutic potential for diabetes and non-alcoholic steatohepatitis. EBioMedicine 63:103202. https://doi.org/10.1016/j.ebiom.2020.103202
Podolsky DK, Gerken G, Eyking A, Cario E (2009) Colitis-associated variant of TLR2 causes impaired mucosal repair because of TFF3 deficiency. Gastroenterology 137(1):209–220. https://doi.org/10.1053/j.gastro.2009.03.007
Shen X, Li Q, Wang F et al (2018) Generation of a novel long-acting thymosin alpha1-Fc fusion protein and its efficacy for the inhibition of breast cancer in vivo. Biomed Pharmacother 108:610–617. https://doi.org/10.1016/j.biopha.2018.09.064
Silvestri C, Pagano E, Lacroix S et al (2020) Fish oil, cannabidiol and the gut microbiota: an investigation in a murine model of colitis. Front Pharmacol 11:585096. https://doi.org/10.3389/fphar.2020.585096
Spalinger MR, Sayoc-Becerra A, Ordookhanian C et al (2021) The JAK inhibitor tofacitinib rescues intestinal barrier defects caused by disrupted epithelial-macrophage interactions. J Crohns Colitis 15(3):471–484. https://doi.org/10.1093/ecco-jcc/jjaa182
Tran CP, Cook GA, Yeomans ND, Thim L, Giraud AS (1999) Trefoil peptide TFF2 (spasmolytic polypeptide) potently accelerates healing and reduces inflammation in a rat model of colitis. Gut 44(5):636–642. https://doi.org/10.1136/gut.44.5.636
Wang W, Zhang D, Yang R et al (2018) Hepatic and cardiac beneficial effects of a long-acting Fc-apelin fusion protein in diet-induced obese mice. Diabetes Metab Res Rev 34(5):e2997. https://doi.org/10.1002/dmrr.2997
Wang F, Cui Y, Shen X, Wang S, Yang GB (2019) IL-17A and IL-17F repair HIV-1 gp140 damaged Caco-2 cell barriers by upregulating tight junction genes. Microbes Infect 21(8–9):393–400. https://doi.org/10.1016/j.micinf.2019.03.003
Wong WM, Poulsom R, Wright NA (1999) Trefoil peptides. Gut 44(6):890–895. https://doi.org/10.1136/gut.44.6.890
Wu MM, Wang QM, Huang BY et al (2021) Dioscin ameliorates murine ulcerative colitis by regulating macrophage polarization. Pharmacol Res 172:105796. https://doi.org/10.1016/j.phrs.2021.105796
Xi Y, Yu D, Yang R et al (2019) Recombinant Fc-Elabela fusion protein has extended plasma half-life andmitigates post-infarct heart dysfunction in rats. Int J Cardiol 292:180–187. https://doi.org/10.1016/j.ijcard.2019.04.089
Xu Z, Chen W, Deng Q et al (2020) Flaxseed oligosaccharides alleviate DSS-induced colitis through modulation of gut microbiota and repair of the intestinal barrier in mice. Food Funct 11(9):8077–8088. https://doi.org/10.1039/d0fo01105c
Zhao S, Wan X, Dai Y, Gong L, Le Q (2022) WNT16B enhances the proliferation and self-renewal of limbal epithelial cells via CXCR4/MEK/ERK signaling. Stem Cell Reports 17(4):864–878. https://doi.org/10.1016/j.stemcr.2022.03.001
Zhou F, Mei J, Han X et al (2019) Kinsenoside attenuates osteoarthritis by repolarizing macrophages through inactivating NF-kappaB/MAPK signaling and protecting chondrocytes. Acta Pharm Sin B 9(5):973–985. https://doi.org/10.1016/j.apsb.2019.01.015
Zimmerman NP, Vongsa RA, Faherty SL, Salzman NH, Dwinell MB (2011) Targeted intestinal epithelial deletion of the chemokine receptor CXCR4 reveals important roles for extracellular-regulated kinase-1/2 in restitution. Lab Invest 91(7):1040–1055. https://doi.org/10.1038/labinvest.2011.77
Funding
This work was supported by the Qingdao National Laboratory for Marine Science and Technology Special Fund of Shandong Province (No. 2022QNLM030003), and the Shandong Program of Qingdao Pilot National Laboratory for Marine Science and Technology (2022QNLM030003-1).
Author information
Authors and Affiliations
Contributions
ML, ZL and JW conceived and designed the experiments; MG, RW, JG and ML performed the experiments; ZL: prepared the TFF2-Fc; ML, MG, RW prepared the manuscript. All authors have read and agreed to the published version of the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors have not disclosed any competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Guo, M., Wang, R., Geng, J. et al. Human TFF2-Fc fusion protein alleviates DSS-induced ulcerative colitis in C57BL/6 mice by promoting intestinal epithelial cells repair and inhibiting macrophage inflammation. Inflammopharmacol 31, 1387–1404 (2023). https://doi.org/10.1007/s10787-023-01226-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10787-023-01226-9