Abstract
Stress is the nonspecific response of the body to any demand made upon it, as defined by Hans Selye more than 80 years ago, based on his animal experiments at McGill University in Montreal, Canada. By emphasizing ‘nonspecificity’ he tried to underline that stress response is elicited my several factors, like nowadays in COVID-19, e.g., fear of infection, social isolation, death in family, loss of employment, etc. Thus, COVID-19 has been the largest new human stressor in the twenty-first century. Selye’s studies in rats also revealed 3 stages of stress response: the short initial “alarm reaction” is followed by a longer “stage of resistance”, associated with increased levels of corticosterone that is often terminated by a “stage of exhaustion”, referring to an exhausted adrenal cortex when the secretion of glucocorticoids drops. Fast forward, that is exactly what has been documented in severe cases of infections caused by the SARS-CoV-2 virus: in hospitalized COVID-19 patients initially the blood levels of cortisol not only have been elevated, but only those with high concentration of this natural anti-inflammatory corticosteroid survived vs. those who had low levels of cortisol, suggesting diminished adrenocortical functions. Furthermore, patients with very severe cases of COVID-19 who ended up in intensive care units had significantly low cortisol blood levels, compared to patients with equal severity of diseases due to other causes. Thus, these ‘natural phenomena’ in clinical medicine, unfortunately confirmed Selye’s studies in experimental animals several decades ago. Still, the good news is that astute clinicians empirically recognized this and started to give potent synthetic glucocorticoids such as dexamethasone to severe COVD-19 patients and this beneficial effect of exogenous corticoids has been extensively confirmed in the scientific literature.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The sudden appearance of COVID-19 at the end of 2019 and early 2020 was a big surprise not only to clinicians but also for public health officials and services in most countries of the world. Based on these, often chaotic, reaction I wrote a review article in mid-2020 on “New disease and chaos with panic, associated with stress” (Szabo 2020), but I could not have predicted that severe COVID-19, with clinical manifestations and molecular mechanisms would so closely resemble the three-stage stress response that Hans Selye described, based on his animal experiments more than 70 years earlier (Selye 1936, 1937, 1976). He also demonstrated that the central organ in stress reactions is the adrenal gland (Selye 1937).
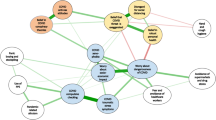
But as more clinical descriptions of COVID-19 started to appear in the literature, and the ‘long COVID syndrome’ manifestations have been documented, it became clear that COVID-19 is the largest new human stressor (i.e., agent that causes stress) in the twenty-first century (Szabo and Zourna 2020). The list of major human stressors (e.g., divorce, unemployment, death in family or close friend, difficulties in work place, arguments with boss) in the last century was compiled by Hans Selye (1976), as his interest shifted from animal experiments to clinical and societal manifestations of stress reactions. Now, that is all compounded by COVID-19, especially since it meets all the requirements for the definition of stress that should be multifactorial in its origin, since “stress is the nonspecific response of the body to any demand made upon it” (Selye 1974). Namely, the stressors in COVID-19 are multiple, e.g., fear of infection, social tensions, with arguments in isolation, reduced income, further aggravated by unemployment or death in family have not only additive, but probably synergistic interactions (Fig. 1). This new human stressor affected first the overworked health care workers, especially nurses and physicians, leading to rapid burnout and resignations from job. However, it also affected parents who had to take care of children, while schools being closed, often leading the ‘broken heart syndrome’ caused by COVID-19 (Fig. 2).
Most of these reports initially were in the popular press, later the scientific literature contained descriptive summaries, but only in late 2021 and early 2022 more analytical, mechanistic studies started to appear. This short review is focused on these recent clinical studies that demonstrated the neuroendocrine mechanisms of what now is named ‘post-COVID stress syndrome’ that strongly resembles the first description of stress response, as Hans Selye demonstrated in rat experiments many years ago.
The first description of three-stage stress response
As mentioned above, the biologic stress response was first characterized based on animal experiments (Selye 1936, 1937) that because of its originality and novelty, the results were published in best scientific journals like Nature and Science. In these experiments, initially performed at McGill University in Montreal, Canada, later continued at University of Montreal when Selye was invited in 1945 to the other big Canadian university, rats were exposed daily to severe physical, chemical, and psychological stressors (e.g., forced immobilization, associated with frustration, subcutaneous injections of small amounts of toxic substances). Autopsy, performed 4 or 7 days after the start of the experiments, revealed a triad of morphologic changes: hyperaemia and enlargement of the adrenal glands, atrophy of thymus and lymphatic glands as well as haemorrhagic gastric erosions and ulcers (Fig. 3).
As a follow-up to these morphologic investigations, later when biochemical methods of measuring the blood concentration of hormones and neurotransmitters became available, experiments revealed elevation of catecholamines, especially epinephrine/adrenaline in the initial stages of stress reaction, that was followed by a sustained elevation of glucocorticoids (i.e., corticosterone in rats and cortisol in human subjects). (Selye 1974, 1976). These structural and functional changes led Selye to define the 3 stages of stress response: Alarm reaction of short duration, followed by a longer stage of resistance, often culminating in exhaustion, when adrenal cortex shrinks, and glucocorticoid levels drop below normal values (Fig. 3).
But what my famous teacher Hans Selye could not have predicted, mostly based on his animal experiments: all this is confirmed in human cases, i.e., sick patients after HIV infection, and more extensively and recently, during the COVD-19 pandemic.
Manifestation of ‘post-COVID stress disorder’
Some of publications refer to this as a “disorder”, while others use the term “syndrome”—which I think is more appropriate. Namely, ‘disorder’ has negative connotations, as something bad, while ‘syndrome’ is more encompassing description of changes happing in severe or prolonged cases of COVID-19. Furthermore, the adrenal changes, elevation of glucocorticoids are defensive reactions, mostly due to the anti-inflammatory effects of this group of natural steroids. Namely, some clinicians knew this much before the recognition of post-COVID stress syndrome when they started to see the beneficial effects of synthetic glucocorticoid such as dexamethasone given to hospitalized COVID-19 patients (Johnson and Vinetz 2020; The RECOVERY Collaborative Group 2021). Actually, one of early editorials very correctly emphasized that “timing is everything” (Johnson and Vinetz 2020) in controlling lung edema and inflammation, since this very potent synthetic steroid may help only before the pathologic pulmonary changes become uncontrollable by the naturally elevated cortisol.
The initial reports on post-COVID stress disorder were just descriptive studies, since the COVID-19 pandemic was triggering a wide variety of emotional, physical and financial economic challenges. Namely, extreme distress in children and adults alike: fear, worry about the future, anger along with symptoms and reactions, such as anxiety, depression, fatigue, difficulty focusing, and problematic behavior. This was usually aggravated by inappropriate use of risk coping mechanisms, such as substance abuse and alcoholism. There was grief over what has been lost and uncertainty about how to navigate daily life, with concerns about what the future holds (Soma 2021).
Some authors compared these reactions and manifestations to PTSD, but it was quickly resolved by emphasizing that PTSD is a traumatic stress reaction to past and largely direct, exposure to certain life-threatening events (Soma 2021. However, the consequences of accumulated distress in COVID-19 aggravated the initial stress reaction, since they increased frustration and despair, led to increased withdrawal, isolation and fear of others as potential source of infection. Furthermore, it also resulted in loss of focus, both on specific tasks and general goals, as well as “fuzzy thinking” or ‘brain fog’ which is the hallmark long COVID syndrome.
As the initial descriptive studies on COVID-associated stress became more analytic and mechanistically oriented, well-documented clinical studies started to show that the initial phases of severe COVID-19 hospitalized patients had elevated cortisol levels (Fig. 4): this was confirmed by an extensive, recent meta-analysis (Amiri-Dashatan et al. 2022). However, as it could have been predicted from the animal experiments of Hans Selye, the adrenal cortex has limited capacity, i.e., started to show decline in very severe cases of hospitalized, especially ICU-bound cases of COVID-19. One of the most convincing case reports (Mao et al. 2021) demonstrates that even in otherwise comparable patients, SARC-CoV-2-infected patients had lower levels of cortisol than other critical patients (Fig. 5), This report concluded that “the cortisol levels are lower in critically ill patients with COVID-19 as compared to those of non-COVID-19 critically ill patients. Based on our findings, we recommend measuring plasma cortisol level to guide hormonal therapy”. Furthermore, not surprisingly, these authors also called attention that the adrenal cortex is “an underestimated site of SARS-CoV-2 infection” (Mao et al. 2021).
One of the most convincing clinical evidence of the three stages of biologic stress response in people comes from the Kaplan–Meier plots of survival probability depending on the blood concentration of cortisol in COVID-19 patients: those who had high cortisol (i.e., remained in the ‘stage of resistance’ with well-functioning adrenal cortex) had statistically significant better survival rates than patients with low levels of cortisol (i.e., were in the ‘stage of exhaustion’ of the adrenal glands) (Fig. 6) (Tan et al. 2020). Needless to say, clinicians who knew about the potent anti-inflammatory action of glucocorticoids and paid attention to the functional stages of adrenal cortex, they soon started to include potent synthetic glucocorticoids (e.g., dexamethasone) in the symptomatic, empirical treatment of severe COVID-19 patients (The RECOVERY Collaborative Group 2021).
Conclusions and discussion
One of the obvious conclusions from this review of historic and recent data from experimental and clinical medicine, respectively, is that basic research not only helps, but leads to a better understanding of the pathogenesis and manifestations of human diseases. Another, early demonstration of the useful transition from animal models of human diseases to applications in clinical medicine is the first use glucocorticoids to treat human inflammatory diseases such as acute rheumatic fever and chronic rheumatoid arthritis in the late 1940s. Namely, Hans Selye demonstrated and published in best scientific journals (e.g., Nature, Science, Endocrinology, Can. Med. Ass. J.) the pro-inflammatory actions of mineralocorticoids and the anti-inflammatory effects of glucocorticoids. Nevertheless, the 1950 Nobel Prize in Physiology or Medicine was awarded to the clinician Philip Hench at the Mayo Clinic while ignoring the original discoverer of these steroid actions by Hans Selye (Szabo et al. 2012).
The limited capacity of the adrenal cortex to synthesize corticosteroids in chronic infectious, inflammatory diseases is not unique to COVID-19. Namely, autopsy reports at the end of twentieth century revealed atrophic adrenal glands in dead HIV-infected patients, and this was best described in one of the largest autopsy reports on AIDS patients: “A morphologic evaluation was carried out on adrenal glands from 128 autopsied patients with the acquired immunodeficiency syndrome (AIDS). The adrenal gland was compromised in 99.2% of the cases, with distinct pathological features and infectious agents. Inflammatory infiltrates were observed in 99.2% of the cases with a predominance of mononuclear cells in 97.4%, affecting mainly the medulla. Necrosis, fibrosis, haemorrhages and neoplasia were also observed” (Rodrigues et al. 2022). Most of these morphologic changes are irreversible and when clinicians saw this kind of reports about 10 years earlier, they started to give cortisol to very sick, hypotensive, often lethargic AIDS patients, and not surprisingly that lethargy disappeared, but the glucocorticoids prolonger the life of these patients by about 6 months (Rodrigues et al. 2022).
It was also predictable that similar findings will be documented when autopsy reports of deceased COVID-19 patients would be published. Indeed, one of the first complete autopsies described the changes in 28 autopsies with confirmed SARS-CoV-2 infection: “Microscopic lesions were identified in the adrenal glands in 12/28 patients (46%). Seven cases showed necrosis, generally ischemic; four showed haemorrhage and one unspecific focal adrenalitis” (Santana et al. 2020). The adrenocortical haemorrhage was so severe that it resembled the Waterhouse–Friderichsen-type adrenal apoplexy in children dying in severe meningococcal sepsis or animal models of this disease (Szabo et al. 1976?).
It should be also underlined that adrenals are not the only endocrine glands affected during acute COVID-19 and the long COVID syndrome. Several short reports and review articles describe the “impact of SARS-CoV-2 virus infection on the endocrine health” (Somasundaram et al. 2020). Furthermore, the London Medical Laboratory reported in early 2022 that “COVID-related stresses have led to a 19% increase in hormone imbalances” that involved not only the adrenals, but pituitary, thyroid, parathyroid and gonads as well. The clinically most noticeable changes involved, after the post-COVID stress syndrome, the destruction of follicular and parafollicular cells in the thyroid as well as of the spermatogonia and seminiferous tubules in the testicles, leading to impaired spermatogenesis and androgen synthesis that results in male hypogonadism and subfertility (Somasundaram et al. 2020).
The molecular and cellular elements in the pathogenesis of these endocrine changes are poorly understood but three components are emerging: (a) since the cellular entry of SARS-CoV-2 is linked to the ACE-2 receptors on cell surface, and since endocrine cells, especially the adrenals, are rich in these receptors, the glands are a major target in acute stages of COVID-19. (b) This postulated mechanism is now documented by advanced in situ hybridization and immunohistochemistry techniques that allow the localization and demonstration of long-term storage of the virus in the endocrines, especially in the adrenal cortex (Kanczkowski et al. 2022). (c) The cytopathic effect of the virus may kill cells, but the massive tissue damage is usually due to microinfarcts that are the result of microthrombi or emboli, as it is demonstrated in the adrenal cortex (Santana et al. 2020).
Thus, the final conclusion could be that COVID-19 has not only and predictably, become the largest, new human stressor in the twenty-first century (Szabo 2020; Szabo and Zourna-Hargaden 2020), but clinical observational and mechanistic studies confirmed the manifestation of the new post-COVID stress syndrome/disorder. Now, ‘only’ the therapeutic options need to be further investigated, since preventive measures have already been suggested (Fig. 7) (Rebello et al. 2022), but their beneficial effects need to be objectively documented.

(Adapted from Rebello et al. 2022)
Exercise may help to reduce post-COVID stress disorder
Data availability
Enquiries about data availability should be directed to the authors.
Abbreviations
- ACE2:
-
Angiotensin converting enzyme 2
- AIDS:
-
Acquired immunodeficiency syndrome
- COVID-19:
-
Coronavirus disease-2019
- HIV:
-
Human immunodeficiency virus
- ICU:
-
Intensive care unit
- PTSD:
-
Post-traumatic stress disorder
- SARS-CoV-2:
-
Severe acute respiratory syndrome-coronavirus 2
References
Amiri-Dashatan N, Koushik M, Parsamanesh N, Chiti H (2022) Serum cortisol concentration and COVID-19 severity—a systemic review and meta-analysis. J Investig Med 70:766–772
Johnson RM, Vinetz JM (2020) Dexamethasone in the management of covid-19. BMJ 370:m2648
Kanczkowski W, Evert K, Stadtmuller M, Haberecker M, Laks L, Chen LS, Frontzek K, Bornstein SR (2022) COVID-19 targets human adrenal glands. Lancet Diabetes Endocrinol 10:13–16
Mao Y, Xu B, Li F, Ren R, Zhu X, Gao Y, Jiang L (2021) The adrenal cortex, an underestimated site of SARS-CoV-2 infection. Front Endocrinol. https://doi.org/10.3389/fendo.2020.593179
Rebello CJ, Axelrod AC, Reynolds CF, Frank L, Greenway FL, Kirwan JP (2022) Exercise as a moderator of persistent neuroendocrine symptoms of COVID-19. Exerc Sport Sci Rev 50(2):65–72
Rodrigues D, Reis M, Teixeira V, Silva-Vergara M (2022) Pathologic findings in the adrenal glands of autopsied patients with acquired immunodeficiency syndrome. Pathol Res Pract 198:25–30
Santana MF, Borba MGS, Baja-de-Silva DC, Val F et al (2020) Case report: Adrenal pathology findings in severe COVID-19. An autopsy study. Am J Trop Med Hyg 103:1604–1607
Selye H (1936) A syndrome produced by diverse nocuous agents. Nature 138:132
Selye H (1937) The significance of the adrenals for adaption. Science 85:247–248
Selye H (1974) Stress without distress. J. B. Lippincott Co., Philadelphia
Selye H (1976) Stress in health and disease. Butterworth’s Inc., Boston
Soma C (2021) Post-COVID stress disorder and pandemic trauma and stress experience. STARR.Org., https://starr.org/2021/post-covid-stress-disorder-and-pandemic-trauma-and-stress-experience/
Somasundaram NP, Ranathunga I, Ratnasamy V, Wijewickrama PSA, Dissanayake HA, Yogendranathan N, Gamage KKK, Lakshitha de Silva N, Sumanatilleke M, Katulanda P, Grossman AB (2020) The impact of SARS-Cov-2 virus infection on the endocrine system. J Endocr Soc. https://doi.org/10.1210/jendso/bvaa082
Szabo S (2020) COVID-19: New disease and chaos with panic, associated with stress. Med Sci 59:41–62. https://doi.org/10.25040/ntsh2020.01.14
Szabo S, Zourna-Hargaden P (2020) COVID-19: new disease and the largest new human stressor. Integr Physiol. https://doi.org/10.33910/2687-1270-2020-1-4
Szabo S, Reynolds ES, Kovacs K (1976) Animal model of human disease. Waterhouse-Friderichsen syndrome. Animal model: acrylonitrile-induced adrenal apoplexy. Am J Pathol 82:653–656
Szabo S, Tache Y, Somogyi A (2012) The legacy of Hans Selye and the origins of stress research: a retrospective 75 years after his landmark “letter” in nature. Stress 15:472–478
Tan T, Khoo B, Mills EG, Phylactou M, Patel B, Eng PC, Thurston L, Muzi B, Meeran K, Prevost AT, Comninos AN, Abbara A, Dhillo WS (2020) Association between high serum total cortisol concentrations and mortality from COVID-19. Lancet Diabetes-Endocrinol 8:659–660
The RECOVERY Collaborative Group (2021) Dexamethasone in hospitalized patients with COVID-19. N Engl J Med 384:693–704
Funding
No grant support was used to prepare this manuscript.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors have not disclosed any competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Szabo, S. The post-COVID stress syndrome: from the three-stage stress response of Hans Selye to COVID-19. Inflammopharmacol 31, 2799–2806 (2023). https://doi.org/10.1007/s10787-023-01179-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10787-023-01179-z