Abstract
We test the effect of the introduction of Medicare Part D on physician prescribing behavior by using data on physician visits from the National Ambulatory Medical Care Survey (NAMCS) 2002–2004 and 2006–2009 for patients aged 60–69. We use regression discontinuity designs to estimate the effect of part D around the age of 65 before and after 2006 and then compare the discrete jump in outcomes at age 65 before and after Part D. We find a 32% increase in the number of prescription drugs prescribed or continued per visit and a 46% increase in the number of generic drugs prescribed or continued for the elderly after the introduction of Medicare Part D.



Similar content being viewed by others
Notes
National health expenditure as a share of GDP grew from about 5% in 1960 to about 17% in 2012. (Source: http://www.cms.gov/Research-Statistics-Data-and-Systems/Statistics-Trends-and-Reports/NationalHealthExpendData/NationalHealthAccountsHistorical.html).
“Physician agency” is a collective term referring to issues related to physicians’ market power, behaviors and incentives (McGuire 2000).
Part D has since become the primary source of drug coverage for Medicare beneficiaries, covering more than half of all beneficiaries (57%) in 2006. It decreased the percentage of Medicare beneficiaries with no source of prescription drug coverage from 27% in 2003 to 10% in 2007 (The Henry J. Kaiser Family Foundation 2003, 2006, 2007b), and lowered the fraction of prescription drug costs that many Medicare beneficiaries had to pay out-of-pocket at the point of service.
Part D has shifted prescription drug costs from Medicaid or private payers to Medicare, and has contributed to a net increase in federal spending. The Medicare share of total national spending on prescription drugs increased from 2% in 2005 to 22% in 2006, and the net federal cost of the Part D program is estimated to be $982 billion for 9 years between 2007 and 2016 (The Henry J. Kaiser Family Foundation 2007a).
Prior work found that advertising of drugs increased only slightly after the introduction of Part D, mostly among drug classes with less competition (defined by the number of advertised drugs within the drug class prior to Part D implementation) or among dominant drugs (defined by higher market share of advertising before the implementation of Part D) (Lakdawalla et al. 2013).
We find little evidence that this may be the case later in the paper.
Direct subsidies reimburse plans for the cost during the initial coverage period; individual reinsurance reimburses plans for the cost during periods of catastrophic coverage; and risk-sharing payments finance unexpectedly high costs to help with plans’ potential losses. The low-income subsidy helps with premium and prescription drug costs for those who are eligible for Medicaid or those with income under 150% of the federal poverty line.
We decide the orders of polynomial for the function of age using two methods. We compare the goodness-of-fit among specifications with different order of polynomial function according to Lee and Lemieux (2010). We also adopt a Wald test to examine the joint significance of additional polynomial terms. The results for both tests are available upon request.
The Charlson index is a numerical score indicating the severity of patient comorbidities. Each condition is associated with a particular score and the scores for each of three possible diagnoses associated with a NAMCS visit are totaled to calculate the index. The Charlson comorbidity index has been shown to predict subsequent mortality (Charlson et al. 1987).
Major disease category includes infectious and parasitic diseases, neoplasms, endocrine, nutritional and metabolic diseases, and immunity disorders, mental disorders, congenital anomalies, as well as diseases of the blood and blood-forming organs, nervous system and sense organs, circulatory system, respiratory system, digestive system, genitourinary system, skin and subcutaneous tissue, musculoskeletal system and connective tissue. Also symptoms, signs, and ill-defined conditions.
Internal Medicine, Pediatrics, General Surgery, Obstetrics & Gynecology, Orthopedic Surgery, Cardiovascular Diseases, Dermatology, Urology, Psychiatry, Neurology, Ophthalmology, Otolaryngology, or other specialties relative to General/Family Practice.
We also estimate the model using Generalized Least Square (GLM) regressions. The results are similar to results we present from OLS regressions.
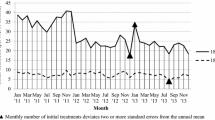
We calculate the number of prescription and generic drugs prescribed or continued by physicians by patient age group and perform a Wald test to examine whether there is a statistically significant difference between the means of outcomes between pair-wise ages and p-values from results to reject the null hypothesis that means are equal.
We also performed GLM regression with a log link and Gaussian distribution, as determined by the Park test. The significance of results from OLS regression still holds and the calculated marginal effects are similar to what we present from OLS regressions.
In results not included in Table 2, we tested whether there was any confounding issue for all other physician and patient characteristics, including physician specialties, patients’ major disease categories and seasonal dummies. None of the coefficients from this set of regressions were significant except for one major disease category: (diseases of the digestive system). Again, excluding these covariates does not affect our main results.
We compute the number of tests by counting how many tests performed or ordered among those recorded in all years of the NAMCS data. These tests included blood pressure check, X-ray, EKG/ECG, Pap test, urinalysis, PSA test, and CBC (complete blood count).
Lichtenberg and Sun (2007) found that in 2006, Medicare Part D reduced OOP daily cost of therapy by 18.4% and increased number of days of therapy by about 12.8% compared to that for users under the age of 65. Yin et al. (2008) find a 5.9% increase in monthly average drug utilization and a 13.1% decrease in monthly OOP cost after the penalty free period (i.e., after June 2006) using those aged 60–63 as the reference group. Ketcham and Simon (2008) estimate that days’ supply and number of individual prescriptions filled increase by 8.1 and 4.8% respectively, and OOP costs fall by 17.2% for the always eligible group (over 66 as of 2007) as compared to those who are always ineligible for Medicare (58–64 as of 2007). Engelhardt and Gruber (2011) find that Part D has a relatively small impact on OOP spending, and F. X. Liu et al. (2011) find that Part D is associated with a $179.86 reduction in OOP cost and an increase of 2.05 prescriptions per patient year. Kaestner and Khan (2012) found that getting drug coverage is associated with an approximately 70% increase in the use of prescription drugs for the general population of those aged 65–85 years old, and a 60% increase in the use of prescription drugs for those with chronic conditions. Briesacher et al. (2011) simulated post-Part D outcomes using time-series regressions with a first-order autoregressive correlation structure and pre-Part D data, and compared those with observed results. They concluded that average prescription fills per person increased significantly by 1.8 fills in 2006 and 3.4 fills in 2007, compared to a 0.9 fill increase per year before Part D. They also found that average OOP drug costs decreased significantly in both 2006 and 2007, by $143 and $148 per year respectively.
The insignificance of our results for some outcomes in the MEPS could result partly from insufficient information in the public use version of the MEPS data to precisely estimate age profiles and time trends of outcomes.
The measure for the utilization of prescription drugs is aggregated to the patient-year level, which is different from the patient-visit level data that are available in NAMCS data for the utilization of prescription drugs. The difference between the estimate from NAMCS and MEPS may be due in part to the fact that different drugs require different day supply amounts and rates of refill.
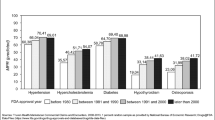
We also tried to merge the FDA data with the NAMCS analytic sample using the whole string in the drug name field. The merge rate was about 80%.
References
Alpert, A. (2012). The anticipatory effects of medicare part D on drug utilization. Journal of Health Economics, 49, 28–45.
Arrow, K. J. (1963). Uncertainty and the welfare economics of medical care. The American Economic Review, 53(5), 941–973.
Blume-Kohout, M. E., & Sood, N. (2013). Market size and innovation: Effects of medicare part D on pharmaceutical research and development. Journal of Public Economics, 97, 327–336.
Briesacher, B. A., et al. (2011). Medicare part D and changes in prescription drug use and cost burden: National estimates for the medicare population, 2000 to 2007. Medical Care, 49(9), 834–841.
Bryant, E. E., & Shimizu, I. (1988). Sample design, sampling variance, and estimation procedure for the national abulatory medical care survey. Vital and Health Statistics. Series 2. Data Evaluation and Methods Research, 108, 1–39.
Card, D., Dobkin, C., & Maestas, N. (2009). Does Medicare Save Lives? The Quarterly Journal of Economics, 124(2), 597–636.
Chalkley, M., & Malcomson, J. M. (1998). Contracting for health services when patient demand does not reflect quality. Journal of Health Economics, 17(1), 1–19.
Charlson, M. E., et al. (1987). A new method of classifying prognostic comorbidity in longitudinal studies: Development and validation. Journal of Chronic Diseases, 40(5), 373–383.
Chay, K.Y., Kim, D. & Swamina, S., 2010. Medicare, hospital utilization and mortality: Evidence from the program’s origins. Working Paper.
Duggan, M., & Morton, F. S. (2010). The effect of medicare part D on pharmaceutical prices and utilization. American Economic Review, 100(1), 590–607.
Duggan, M. G., & Morton, F. S. (2011). The medium-term impact of medicare part D on pharmaceutical prices. American Economic Review, 101(3), 387–392.
Ellis, R. P., & McGuire, T. G. (1986). Provider behavior under prospective reimbursement. Journal of Health Economics, 5(2), 129–151.
Engelberg, J., Parsons, C. A., & Tefft, N. (2013). First, do no harm: Financial conflicts in medicine. Working Paper.
Engelhardt, G. V., & Gruber, J. (2011). Medicare Part D and the financial protection of the elderly. American Economic Journal: Economic Policy, 3(4), 77–102.
Epstein, A. J., & Ketcham, J. D. (2014). Information technology and agency in physicians’ prescribing decisions. The RAND Journal of Economics, 45(2), 422–448.
Federman, A. D. (2004). Don’t ask, don’t tell: The status of doctor-patient communication about health care costs. Archives of Internal Medicine, 164(16), 1723–1724.
Feldstein, M. S. (1970). The rising price of physicians’ services. The Review of Economics and Statistics, 52(2), 121–133.
Grembi, V., Nannicini, T., & Troiano, U. (2011). Do fiscal rules matter? A difference-in-discontinuities design. Working Paper.
Imbens, G. W. (2008). Regression discontinuity designs: A guide to practice. Journal of Econometrics, 142(2), 615–635.
Kaestner, R., & Khan, N. (2012). Medicare part D and its effect on the use of prescription drugs and use of other health care services of the elderly. Journal of Policy Analysis and Management, 31(2), 253–279.
Kaestner, R., Long, C., & Alexander, G. C. (2014). Effects of prescription drug insurance on hospitalization and mortality: Evidence from medicare part D. NBER Working Paper Series, (19948).
Ketcham, J. D., & Simon, K. I. (2008). Medicare part D’s effects on elderly patients’ drug costs and utilization. The American Journal of Managed Care, 14(11 Suppl), SP14–SP22.
Lakdawalla, D., Sood, N., & Gu, Q. (2013). Pharmaceutical Advertising and Medicare Part D. Journal of Health Economics, 32(6), 1356–67.
Lee, D. S., & Lemieux, T. (2010). Regression discontinuity designs in economics. Journal of Economic Literature, 48(2), 281–355.
Lichtenberg, F. R., & Sun, S. X. (2007). The impact of medicare part D on prescription drug use by the elderly. Health affairs (Project Hope), 26(6), 1735–1744.
Liu, F. X., et al. (2011). The impact of medicare part D on out-of-pocket costs for prescription drugs, medication utilization, health resource utilization, and preference-based health utility. Health Services Research, 46(4), 1104–1123.
Lurie, N., et al. (1990). Pharmaceutical Representatives in academic medical centers: Interaction with faculty and housestaff. Journal of General Internal Medicine, 5(3), 240–243.
McCrary, J. (2008). Manipulation of the running variable in the regression discontinuity design: A density test. Journal of Econometrics, 142(2), 698–714.
McGuire, T. G. (2000). Physician agency. Handbook of Health Economics, 1, 461–536.
Meyers, D. S., et al. (2006). Primary care physicians’ perceptions of the effect of insurance status on clinical decision making. Annals of Family Medicine, 4(5), 399–402.
Sacks D.W. (2013). Physician agency, compliance, and patient welfare?: Evidence from anti-cholesterol drugs. Working Paper.
The Henry J. Kaiser Family Foundation (2003). The medicare prescription drug benefit fact sheet,
The Henry J. Kaiser Family Foundation (2006). The medicare prescription drug benefit fact sheet,
The Henry J. Kaiser Family Foundation (2007a). Medicare spending and financing fact sheet,
The Henry J. Kaiser Family Foundation (2007b). The medicare prescription drug benefit fact sheet
The Henry J. Kaiser Family Foundation (2010). The medicare prescription drug benefit fact sheet.
Weisbrod, B. A. (1991). The health care quadrilemma: An essay on technological change, insurance, quality of care, and cost containment. Journal of Economic Literature, 29(2), 523–552.
Wynia, M. K., et al. (2003). Do physicians not offer useful services because of coverage restrictions? Health Affairs (Project Hope), 22(4), 190–197.
Yin, W., et al. (2008). The effect of the medicare part D prescription benefit on drug utilization and expenditures. Annals of Internal Medicine, 148(3), 169–177.
Zhang, Y., et al. (2009). The effect of medicare part D on drug and medical spending. The New England Journal of Medicine, 361(1), 52–61.
Author information
Authors and Affiliations
Corresponding author
Additional information
Tianyan Hu: I thank Mary E. Deily, Thomas Buchmueller and participants at the 2012 Eastern Economic Association Annual Meeting, 2012 American Society of Health Economists conference, 2016 American Society of Health Economists Conference, and seminars at Harvard University, University of Pennsylvania, the Johns Hopkins University and the National Center for Health Statistics, CDC. Sandra Decker: The majority of this work was done while Sandra Decker was a distinguished consultant at the National Center for Health Statistics of the Centers for Disease Control and Prevention (CDC). The views expressed in this article are those of the authors and do not necessarily represent those of CDC or the Agency for Healthcare Research and Quality.
Appendix: Description of the data-merging procedure
Appendix: Description of the data-merging procedure
The FDA drug approval database was retrieved from: http://www.fda.gov/Drugs/InformationOnDrugs/ucm129689.htm (August, 2011).
Three zipped ASCII files were downloaded from the website. The one under the name products.txt was the one containing information about ingredient, dosage form, trade name, approval date and type (whether a prescription drug or not). We transferred the data into Stata format using StatTransfer. We cleaned the data to calculate the approval date for each drug, counting drugs with different manufacturers or strengths or packages as the same drug.
The NAMCS data has variables called MED1-MED8 (up to MED6 if before 2003). These variables are coded using a different coding system beginning in 2006, but with the SAS program (DRUGCHAR_MULTUM_‘year’.sas) provided available from NCHS, one can update the coding for variables from NAMCS 2002–2005 so that they are coded as are the variables for 2006 and forward. We then identify the drug trade name using appendix B (drug entry codes and names in numeric order) from the FDA site. We imported data into Stata and merged this list with NAMCS data.
This resulted in the drug trade names appearing in both the FDA and NAMCS datasets, so we could then merge the FDA approval date with the NAMCS data using the first word of the drug name. (Most of the drugs can be identified with the first word in its name). The merge rate for drugs prescribed in the NAMCS drug sample was 95% with this method.Footnote 22
Rights and permissions
About this article
Cite this article
Hu, T., Decker, S.L. & Chou, SY. The impact of health insurance expansion on physician treatment choice: Medicare Part D and physician prescribing. Int J Health Econ Manag. 17, 333–358 (2017). https://doi.org/10.1007/s10754-017-9211-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10754-017-9211-2