Abstract
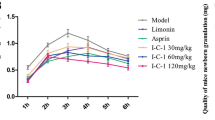
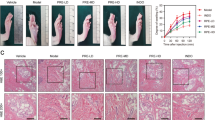
Rhoifolin (ROF) is a main effective component in Citrus grandis ‘Tomentosa’. ROF has a potential anti-inflammatory activity, but its specific effects and mechanisms have not been studied. This study investigated the anti-inflammatory activity of ROF and searched for its possible molecular mechanisms. A mouse model of acute inflammation was induced by lipopolysaccharide, and the effects of ROF on pathological damages of the lung and liver were observed. Carrageenan-induced paw edema rat model was used to evaluate the effect of ROF on the volume of swelling paw. In LPS-induced RAW264.7 macrophages, the expression levels of pro-inflammatory cytokines IL-1β, IL-6, and TNF-α were measured using ELISA. Real-time PCR was used to measure the mRNA levels of iNOS and CCL2. Western blot was used to detect the activation of IκBα and IKKβ in NF-κB signaling pathways. The results showed that ROF accelerated the recoveries of liver and lung tissue damages in acute inflammation mice and inhibited carrageenan-induced paw edema in rats; in addition, ROF significantly suppressed the secretion of TNF-α, IL-1β, and IL-6 in the serum of rats and mouse model. In LPS-induced RAW264.7 cells, 100 μmol/L ROF enhanced cell viability and suppressed the production of TNF-α, IL-6, and IL-1β significantly. ROF also decreased the mRNA expression of iNOS and CCL2 and inhibited IκBα and IKKβ phosphorylation. In summary, ROF had a potential therapeutic value for inflammation. Our research provided experimental basis for the further development of ROF as an anti-inflammatory drug and for clarifying the anti-inflammatory substance basis of Citrus grandis ‘Tomentosa’.

Graphical Abstract





Similar content being viewed by others
References
Liu, M.-H., H.-B. Liu, W.-W. Su, X.-R. Liu, and J.-H. Li. 2012. Study on impurities in naringin extracted from Citrus grandis “Tomentosa”. Zhong Yao Cai 35: 56–61 http://www.ncbi.nlm.nih.gov/pubmed/22734411.
Ma, S.G., R.B. Wang, W.R. Li, Y.B. Liu, J. Qu, Y. Li, X.J. Wang, S. Xu, and S.S. Yu. 2018. New C-2 diastereomers of flavanone glycosides conjugated with 3-hydroxy-3-methylglutaric acid from the pericarp of Citrus grandis (L.) Osbeck. Bioorganic Chemistry 80: 519–524. https://doi.org/10.1016/j.bioorg.2018.06.024.
Weiwei, Su. 2001. Research progress of Exocarpium Citri grandis, research progress of chemical orange red [J]. Chinese Medicinal Materials 08: 608–610.
Barreca, D., E. Bellocco, C. Caristi, U. Leuzzi, and G. Gattuso. 2010. Flavonoid composition and antioxidant activity of juices from Chinotto (Citrus x myrtifolia Raf.) fruits at different ripening stages. Journal of Agricultural and Food Chemistry 58: 3031–3036. https://doi.org/10.1021/jf9044809.
Kuo, P.C., Y.R. Liao, H.Y. Hung, C.W. Chuang, T.L. Hwang, S.C. Huang, Y.J. Shiao, D.H. Kuo, and T.S. Wu. 2017. Anti-inflammatory and neuroprotective constituents from the peels of citrus grandis. Molecules. 22: 1–11. https://doi.org/10.3390/molecules22060967.
Tian, D., F. Wang, M. Duan, L. Cao, Y. Zhang, X. Yao, and J. Tang. 2019. Coumarin analogues from the Citrus grandis (L.) Osbeck and their hepatoprotective activity. Journal of Agricultural and Food Chemistry 67: 1937–1947. https://doi.org/10.1021/acs.jafc.8b06489.
Eldahshan, O.A., and S.S. Azab. 2012. Anti-inflammatory effect of apigenin-7-neohesperidoside (rhoifolin) in carrageenin-induced rat oedema model. Journal of Applied Pharmaceutical Science 2: 74–79. https://doi.org/10.7324/JAPS.2012.2811.
Eldahshan, O. 2014. Rhoifolin; A potent antiproliferative effect on cancer cell lines. British Journal Pharmaceutical Research 3: 46–53. https://doi.org/10.9734/bjpr/2013/1864.
Gordon, P.B., I. Holen, and P.O. Seglen. 1995. Protection by naringin and some other flavonoids of hepatocytic autophagy and endocytosis against inhibition by okadaic acid. The Journal of Biological Chemistry 270: 5830–5838. https://doi.org/10.1074/jbc.270.11.5830.
X. Song, L. Han, et al. Li, Yubang, The anti-inflammatory effect and mechanism of rhoifolin on LPS-induced RAW264.7 cells, Journal of Henan Normal University (Natural Science) 2018, 46 (02): 84–88+94. https://doi.org/10.16366/j.cnki.1000-2367.2018.02.013
Jingran Lin. Extraction and pharmacokinetics of rhoifolin in Citrus grandis ‘Tomentosa’ [D] .Guangzhou University of Traditional Chinese Medicine, 2014.
Xiao Lu. Protective effect of anti-inflammatory coagulation effect of low molecular weight heparin on acute lung injury in sepsis rats [D] .Second Military Medical University, 2010
Su, J.Y., L.R. Tan, P. Lai, H.C. Liang, Z. Qin, M.R. Ye, X.P. Lai, and Z.R. Su. 2012. Experimental study on anti-inflammatory activity of a TCM recipe consisting of the supercritical fluid CO2 extract of Chrysanthemum indicum, Patchouli oil and Zedoary turmeric oil in vivo. Journal of Ethnopharmacology 141: 608–614. https://doi.org/10.1016/j.jep.2011.08.055.
Lee, H.C., F.C. Liu, C.N. Tsai, A.H. Chou, C.C. Liao, and H.P. Yu. 2020. Esculetin ameliorates lipopolysaccharide-induced acute lung injury in mice via modulation of the AKT/ERK/NF-κB and RORγt/IL-17 pathways. Inflammation. https://doi.org/10.1007/s10753-020-01182-4.
Zhang, Y., Y. Li Yu, H. Tian, R. Yu Bai, Y. Nan Bi, X. Mei Yuan, L. Kang Sun, Y. Ru Deng, and K. Zhou. 2019. Evaluation of anti-inflammatory activities of a triterpene β-elemonic acid in frankincense in vivo and in vitro. Molecules. 24: 1–13. https://doi.org/10.3390/molecules24061187.
Bradley, J. 2008. TNF-mediated inflammatory disease. The Journal of Pathology 214: 149–120. https://doi.org/10.1002/path.2287.
Huang, X., Y. You, Y. Xi, B. Ni, X. Chu, R. Zhang, and H. You. 2020. p-Coumaric acid attenuates IL-1β-induced inflammatory responses and cellular senescence in rat chondrocytes. Inflammation 43: 619–628. https://doi.org/10.1007/s10753-019-01142-7.
Gu, L., B. Rutledge, J. Fiorillo, C. Ernst, I. Grewal, R. Flavell, R. Gladue, and B. Rollins. 1997. In vivo properties of monocyte chemoattractant protein-1. Journal of Leukocyte Biology 62: 577–580. https://doi.org/10.1002/jlb.62.5.577.
Kolattukudy, P.E., and J. Niu. 2012. Inflammation, endoplasmic reticulum stress, autophagy, and the monocyte chemoattractant protein-1/CCR2 pathway. Circulation Research 110: 174–189. https://doi.org/10.1161/circresaha.111.243212.
Joung, E.J., B. Lee, W.G. Gwon, T. Shin, B.M. Jung, N.Y. Yoon, J.S. Choi, C.W. Oh, and H.R. Kim. 2015. Sargaquinoic acid attenuates inflammatory responses by regulating NF-κB and Nrf2 pathways in lipopolysaccharide-stimulated RAW 264.7 cells. International Immunopharmacology 29: 693–700. https://doi.org/10.1016/j.intimp.2015.09.007.
Sheikine, Y., and G.K. Hansson. 2004. Chemokines and atherosclerosis. Annals of Medicine 36: 98–118. https://doi.org/10.1080/07853890310019961.
Moalem, G., and D.J. Tracey. 2006. Immune and inflammatory mechanisms in neuropathic pain. Brain Research Reviews 51: 240–264. https://doi.org/10.1016/j.brainresrev.2005.11.004.
Janakiram, N.B., and C.V. Rao. 2012. INOS-selective inhibitors for cancer prevention: promise and progress. Future Medicinal Chemistry 4: 2193–2204. https://doi.org/10.4155/fmc.12.168.
Wang, P.-C., S. Zhao, J. Feng, H. Zhao, Q.-H. Wang, and H.-X. Kuang. 2015. Advances in studies on Chinese materia medica for treatment of ulcerative colitis based on NF-κB signal pathway. Chinese Traditional and Herbal Drugs 46: 1556–1561. https://doi.org/10.7501/j.issn.0253-2670.2015.10.026.
Ham, Y., Y. Ko, S. Song, J. Kim, K. Kim, J. Yun, J. Cho, G. Ahn, and W. Yoon. 2015. Anti-inflammatory effect of litsenolide B2 isolated from Litsea japonica fruit via suppressing NF- κ B and MAPK pathways in LPS-induced RAW264.7 cells. Journal of Functional Foods 13: 80–88. https://doi.org/10.1016/j.jff.2014.12.031.
Funding
This study was supported by Chinese medicine standardization project from the National Development and Reform Commission and National Administration of Traditional Chinese Medicine (ZYBZH-Y-GD-13), Science Program for Overseas Scholar of Guangzhou University of Chinese Medicine (Torch Program) (XH20170109), and the Innovation Team Construction Project with Agricultural Products as the Unit of Modern Agricultural Industry Technology System of Guangdong Province (2019KJ148).
Author information
Authors and Affiliations
Contributions
Concept and design: Fengxia Xiao and Xiaoxin Song; data collection: Xiaoxin Song and Zelin Cao; data analysis and interpretation: Jiaqi Fang and Xiaoxin Song; generating the manuscript: Jiaqi Fang, Taojunfeng Su, Yuguang Chi, Baoyu Mai, Tingfang Wen, Jingran Lin, Xiaoying Zhang, and Jialan Chen
Corresponding author
Ethics declarations
Competing Interests
The authors declare that they have no conflict of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Fang, J., Cao, Z., Song, X. et al. Rhoifolin Alleviates Inflammation of Acute Inflammation Animal Models and LPS-Induced RAW264.7 Cells via IKKβ/NF-κB Signaling Pathway. Inflammation 43, 2191–2201 (2020). https://doi.org/10.1007/s10753-020-01286-x
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10753-020-01286-x