Abstract
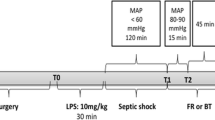
Shock is associated with inflammation-induced endothelial dysfunction. The aim of this study was to determine time-dependent alteration of blood biomarkers related to endothelial function in hemorrhagic and septic shocks. Hemorrhagic shock was induced by bleeding the animals. A cecal ligation and incision model was used to induce septicemia. Resuscitation was carried out by infusion of lactated Ringer’s solution. Resuscitation extended survival time in both shock groups. Blood pressure increased by resuscitation in the hemorrhagic shock but not in the septic shock. While hemorrhage caused a decrease in plasma levels of nitric oxide (NO) and hydrogen sulfide (H2S), asymmetric dimethylarginine (ADMA) and total antioxidant capacity (TAC) levels were increased. Only NO and TAC levels at the late phase were reversed by resuscitation. On the other hand, plasma levels of NO, ADMA, and TAC were increased by septicemia and resuscitation did not alter the septicemia-induced increase. These results indicate that blood biomarkers related to endothelial function were differentially affected by hemorrhage and septicemia. The time scale of biomarker production should be taken into consideration for the diagnostic and therapeutic approaches to these life-threatening diseases.






Similar content being viewed by others
References
Kobayashi, L., T.W. Costantini, and R. Coimbra. 2012. Hypovolemic shock resuscitation. The Surgical Clinics of North America 92 (6): 1403–1423.
Dellinger, R.P., M.M. Levy, A. Rhodes, Surviving Sepsis Campaign Guidelines Committee including the Pediatric Subgroup, et al. 2013. Surviving sepsis campaign: International guidelines for management of severe sepsis and septic shock: 2012. Critical Care Medicine 41 (2): 580–637.
Paulus, P., C. Jennewein, and K. Zacharowski. 2011. Biomarkers of endothelial dysfunction: Can they help us deciphering systemic inflammation and sepsis? Biomarkers Suppl 1: S11–S21.
Tunctan, B., B. Korkmaz, A.N. Sari, et al. 2012. A novel treatment strategy for sepsis and septic shock based on the interactions between prostanoids, nitric oxide, and 20-hydroxyeicosatetraenoic acid. Antiinflamm Antiallergy Agents Med Chem 11 (2): 121–150.
Zhang, H., S.M. Moochhala, and M. Bhatia. 2008. Endogenous hydrogen sulfide regulates inflammatory response by activating the ERK pathway in polymicrobial sepsis. Journal of Immunology 181: 4320–4331.
Ait-Oufella, H., E. Maury, S. Lehoux, et al. 2010. The endothelium: Physiological functions and role in microcirculatory failure during severe sepsis. Intensive Care Medicine 36 (8): 1286–1298.
Lundy, D.J., and S. Trzeciak. 2011. Microcirculatory dysfunction in sepsis. Critical Care Nursing Clinics of North America 23 (1): 67–77.
Szabó, C., and C. Thiemermann. 1994. Invited opinion: Role of nitric oxide in hemorrhagic, traumatic, and anaphylactic shock and thermal injury. Shock 2 (2): 145–155.
Shah, N.S., and T.R. Billiar. 1998. Role of nitric oxide in inflammation and tissue injury during endotoxemia and hemorrhagic shock. Environmental Health Perspectives Supplements 5: 1139–1143.
Förstermann, U., and W.C. Sessa. 2012. Nitric oxide synthases: Regulation and function. European Heart Journal 33 (7): 829–837.
Szabó, C., and K. Módis. 2010. Pathophysiological roles of peroxynitrite in circulatory shock. Shock Suppl 1: 4–14.
Andrades, M.É., A. Morina, S. Spasić, et al. 2011. Spasojević I. Bench-to-bedside review: Sepsis—from the redox point of view. Critical Care 15 (5): 230.
Koch, A., R. Weiskirchen, J. Kunze, et al. 2013. Elevated asymmetric dimethylarginine levels predict short- and long-term mortality risk in critically ill patients. Journal of Critical Care 28 (6): 947–953.
Van de Louw, A., and P. Haouzi. 2012. Oxygen deficit and H2S in hemorrhagic shock in rats. Critical Care 16 (5): R178.
Scheiermann, P., S. Hoegl, M. Revermann, et al. 2009. Cecal ligation and incision: An acute onset model of severe sepsis in rats. The Journal of Surgical Research 151 (1): 132–137.
Wang, P., and I.H. Chaudry. 1998. A single hit model of polymicrobial sepsis: Cecal ligation and puncture. Sepsis 2: 227–233.
Navarro-Gonzalvez, J., C. Garcia-Benayas, and J. Arenas. 1998. Semiautomated measurement of nitrate in biological fluids. Clinical Chemistry 44 (3): 679–681.
Usanmaz, S.E., and E. Demirel-Yilmaz. 2008. A microplate based spectrophotometric method for the determination of the total antioxidant capacity of human plasma: Modified cupric reducing ability assay. Fundamental & Clin Pharmacol 22 (Suppl .2): 67–67.
Hua, T.C., and S.M. Moochhala. 2000. Role of nitric oxide in hemorrhagic shock-induced bacterial translocation. The Journal of Surgical Research 93 (2): 247–256.
Ng, K.C., S.M. Moochhala, S. Md, E.L. Yap, S.Y. Low, and J. Lu. 2003. Preservation of neurological functions by nitric oxide synthase inhibitors following hemorrhagic shock. Neuropharmacology 44 (2): 244–252.
Savage, S.A., C.M. Fitzpatrick, V.S. Kashyap, et al. 2005. Endothelial dysfunction after lactated Ringer’s solution resuscitation for hemorrhagic shock. The Journal of Trauma 59 (2): 284–290.
Douzinas, E.E., O. Livaditi, A.G. Xiarchos, et al. 2006. The effect of hypoxemic resuscitation of hemorrhagic shock on hemodynamic stabilization and inflammatory response: A pilot study in a rat experimental model. The Journal of Trauma 61 (4): 918–923.
Shih, C.C., S.J. Chen, A. Chen, et al. 2008. Therapeutic effects of hypertonic saline on peritonitis-induced septic shock with multiple organ dysfunction syndrome in rats. Critical Care Medicine 36 (6): 1864–1872.
Chen, K., R.N. Pittman, and A.S. Popel. 2009. Hemorrhagic shock and nitric oxide release from erythrocytic nitric oxide synthase: A quantitative analysis. Microvascular Research 78 (1): 107–118.
Barmaki, B., A. Nasimi, and M. Khazaei. 2011. Effects of hypertension on hemodynamic response and serum nitrite concentration during graded hemorrhagic shock in rats. J Res Med Sci 16 (9): 1168–1175.
Subeq, Y.M., B.G. Hsu, N.T. Lin, et al. 2012. Hypothermia caused by slow and limited-volume fluid resuscitation decreases organ damage by hemorrhagic shock. Cytokine 60 (1): 68–75.
Victor, V.M., M. Rocha, and M. De la Fuente. 2004. Immune cells: Free radicals and antioxidants in sepsis. International Immunopharmacology 4 (3): 327–347.
Aneman, A., V. Backman, J. Snygg, et al. 1994. Accumulation of an endogenous inhibitor of nitric oxide synthase during graded hemorrhagic shock. Circulatory Shock 44 (3): 111–114.
Balabanli, B., H. Erdamar, N. Türközkan, et al. 2007. Effect of taurine on endotoxin-induced alterations in plasma asymmetric dimethylarginine, L-arginine and nitric oxide in guinea pigs. Journal of Thrombosis and Thrombolysis 24 (1): 53–57.
Mok, Y.Y., M.S. Atan, C. Yoke Ping, et al. 2004. Role of hydrogen sulphide in haemorrhagic shock in the rat: Protective effect of inhibitors of hydrogen sulphide biosynthesis. British Journal of Pharmacology 143 (7): 881–889.
Li, L., M. Bhatia, Y.Z. Zhu, et al. 2005. Hydrogen sulfide is a novel mediator of lipopolysaccharide-induced inflammation in the mouse. The FASEB Journal 19 (9): 1196–1198.
Zhang, H., L. Zhi, P.K. Moore, and M. Bhatia. 2006. Role of hydrogen sulfide in cecal ligation and puncture-induced sepsis in the mouse. American Journal of Physiology. Lung Cellular and Molecular Physiology 290 (6): L1193–L1201.
Acknowledgements
The present study was supported by a grant from The Commission of the Scientific Research Projects of Uludag University (2008/43). We are grateful to Soner Mamuk for the great technical assistance.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that there are no conflict of interest.
Rights and permissions
About this article
Cite this article
Coskun, C.N., Usanmaz, S.E., Savci, V. et al. Time-Dependent Production of Endothelium-Related Biomarkers is Affected Differently in Hemorrhagic and Septic Shocks. Inflammation 41, 33–41 (2018). https://doi.org/10.1007/s10753-017-0660-z
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10753-017-0660-z