Abstract
Sepsis is a life-threatening condition characterised by endothelial barrier dysfunction and impairment of normal microcirculatory function, resulting in a state of hypoperfusion and tissue oedema. No specific pharmacological therapies are currently used to attenuate microvascular injury. Given the prominent role of endothelial breakdown and microcirculatory dysfunction in sepsis, there is a need for effective strategies to protect the endothelium. In this review we will discuss key mechanisms and putative therapeutic agents relevant to endothelial barrier function.
Similar content being viewed by others
Introduction
Sepsis is a state of organ dysfunction caused by a dysregulated host immune response to infection [1]. Despite advances in medical care, sepsis remains a leading cause of death, accounting for more than 20% of global deaths [2]. A hallmark feature of sepsis is microcirculatory dysfunction which manifests as areas of heterogenous or absent blood flow due to dysregulation of vascular tone, shunting of blood directly from arterioles to venules, and microthromboses [3]. Another key feature of sepsis is enhanced endothelial permeability which leads to interstitial oedema [4]. While this initial increased endothelial permeability is likely beneficial to the host immune response by allowing the transvascular flux of antibodies and antibacterial peptides, ultimately this becomes harmful [4, 5].
Endothelial dysfunction is a common feature of acute inflammatory disorders including burns, trauma, and acute respiratory distress syndrome (ARDS) including that caused by COVID-19, as well as sepsis, and may account for overlap in clinical features between these syndromes.
Endothelial structure and function
The vascular tree is lined by a monolayer of endothelial cells which are critical to vascular integrity, haemostasis, vasomotor control, and immunological defence via exocrine, paracrine, and autocrine actions [6, 7]. The luminal surface is coated with the endothelial glycocalyx, a gel-like matrix of proteoglycans and glycoproteins [8]. In humans, estimates of endothelial surface area vary between 270 and 7000 m2 [9, 10].
A key mediator of vascular tone is nitric oxide (NO), which is synthesised in endothelial cells [11]. NO production is modulated by endothelial shear stress and by various signalling molecules, such as bradykinin, adenosine, serotonin, and vascular endothelial growth factor (VEGF) [12, 13]. Due to the pervasive role of dysregulated NO activity in sepsis, many attempts have been made to correct the heterogenous imbalance of NO in sepsis, all of which have failed to demonstrate benefit [14,15,16,17].
Endothelial cells also produce prostacyclin which, in addition to contributing to vasodilation, prevents platelet deposition on the vessel wall [18]. The endothelium produces potent vasoconstrictors such as Endothelin-1 [19] and facilitates the conversion of Angiotensin-1 into Angiotensin-2, another potent vasoconstrictor which is a product of the renin–angiotensin–aldosterone system [20].
Endothelial cell–cell junctions
Complex inter-endothelial junctional structures, such as adherens junctions and tight junctions, perform a critical role in maintaining vascular integrity and allow endothelial cells to communicate with surrounding structures. The organisation of endothelial cell–cell junction complexes varies along the vascular tree [21]—for example, endothelial junctions in the brain are rich in tight junctions which ensure strict control of permeability across the blood brain barrier [22]. This contrasts with poorly organised tight junctions located in postcapillary venules which readily permit extravasation of inflammatory and immune cells [21, 23].
Adherens junctions are responsible for regulation of cell–cell adhesion, the actin cytoskeleton and intra-cellular signalling [24] and are composed of the core transmembrane protein vascular-endothelial (VE)-cadherin which interacts with cytoplasmic proteins known as catenins. In sepsis, the extracellular domain of VE-cadherin is subject to proteolysis by neutrophil elastase [25] and metalloproteinases [26].
VE-cadherin junctions are tightly regulated by Rho proteins, a subfamily of small GTPases which belong to the Ras superfamily [27]. Key subtypes of the Rho subfamily include Rac1 and RhoA which have been identified to perform central roles in the maintenance of endothelial barrier integrity. The carefully balanced activation of Rac1 and inhibition of RhoA stabilises the VE-cadherin complex and prevents vascular leakage [28]. In experimental models of sepsis, this balance is lost, and impairment of Rho-associated pathways has been identified in endothelial cells [27]. Rac1 activation and RhoA inhibition are associated with VE-cadherin stabilisation and reduced vascular leakage in lipopolysaccharide (LPS) and interleukin (IL)-1β models of endothelial dysfunction [29, 30].
Tight junctions serve to form a continuous intercellular barrier between cells and act to control the paracellular movement of ions and solutes [24, 31]. Tight junctions are composed of adhesion molecules, such as claudin, occludin and junction adhesion molecules, which exist in complex with the cytoplasmic scaffolding proteins zonula occludens (ZO)-1,-2 and -3 (Fig. 1) [24, 32]. The ZO scaffolding proteins link tight junctions to the actin cytoskeleton either through a direct link or through further protein interactions [24]. ZO-1 has multiple domains which permit a wide array of cellular signalling, thereby providing plasticity of tight junction function [33, 34].
Endothelial cell–cell junction complexes. These key junctional structures maintain endothelial barrier integrity. The ZO proteins link the membrane proteins to the filamentous cytoskeleton. Members of the Rho family of GTPases mediate opposing changes in endothelial cell permeability with Rac1 stabilising the VE-cadherin complex and RhoA de-stabilising the VE-cadherin complex
In addition to the key role of adherens junctions and tight junctions in maintaining vascular homeostasis, connexins perform a vital role in intercellular communication. Connexins are transmembrane proteins which form intercellular channels and connect the cytoplasms of adjacent cells, thereby allowing the exchange of ions and small metabolites [35].
Disruption of key adhesion molecules is mediated by TNF-α and IL-1β, key pro-inflammatory cytokines in sepsis, whose production is increased as a result of activation of NF-κB dependent transcription [36]. In septic patients, NF-κB activity correlates with the severity of illness and is significantly higher in non-survivors [37]. NF-κB activation performs a crucial role in the pathophysiology of sepsis by mediating the inflammatory response via the production of key cytokines, such as TNF-α (Fig. 2) [38].
An array of microbial components stimulate the innate immune response by activating Toll-like receptors which results in the nuclear translocation of the transcription factor NF-κB. NF-κB then promotes the expression of pro-inflammatory cytokines such as TNF- α which induces endothelial cell dysfunction
In experimental models of sepsis, the NF-κB pathway is stimulated with the use of LPS, a component of the outer membrane of Gram-negative bacteria [39]. LPS performs a key role in driving Gram negative sepsis [40, 41] by activating Toll-like receptor (TLR) signalling. Ultimately, this cascade enables the nuclear translocation of key transcription factors, such as NF-kB in order to promote pro-inflammatory cytokine gene transcription [42, 43].
TNF-α is perhaps the most extensively studied pro-inflammatory cytokine. Tracey and colleagues confirmed that the administration of recombinant TNF-α can induce shock and tissue injury [44]. Moreover, it has been demonstrated that the administration of anti-TNF antibodies could prevent shock, organ dysfunction and death in a baboon Escherichia coli model of sepsis [45]. However, despite promising pre-clinical evidence the use of anti-TNF-α therapies has proven disappointing in clinical trials [46, 47].
VEGF is a potent angiogenesis factor and pro-permeability mediator which is produced by endothelial cells and macrophages among a variety of cell types [48]. VEGF expression is primarily promoted by hypoxia [49], but also by pro-inflammatory cytokines such as IL-1 [50], IL-1β and TNF-α [51]. VEGF is thought to promote endothelial cell permeability via a range of mechanisms. Firstly, it has been demonstrated that the treatment of endothelial cells with VEGF results in the development of, previously absent, fenestrations [52, 53]. Secondly, VEGF results in the formation of clusters of vesicles which link the luminal and abluminal surfaces of endothelial cells. These clusters have been termed vesicular vacuolar organelles and are thought to form a pathway for the transcellular movement of fluid and solute [54, 55]. Finally, VEGF may directly interfere with key endothelial junctional structures. Using immunofluorescence based techniques Kevil and colleagues revealed that endothelial cell treatment with VEGF resulted in a loss of VE-cadherin and occludin [56].
Endothelial glycocalyx
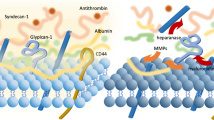
The glycocalyx, a mesh-like network of proteoglycans and glycoproteins, lines the vascular endothelium [57], and regulates capillary and interstitial oncotic pressures to modulate fluid filtration [58, 59]. Restriction of the transvascular movement of large, negatively-charged molecules such as albumin results in an albumin gradient which opposes fluid flux across the endothelium [60].
In sepsis, degeneration of the glycocalyx results in vascular leak, impaired perfusion, aberrant coagulation and leucocyte activation and adhesion [61,62,63]. This glycocalyceal degeneration is mediated by sheddases, enzymes such as heparinase and metalloproteinases, which are activated by inflammatory cytokines, such as TNF-α, and by Reactive Oxygen Species (ROS) [64, 65], and which cleave the key glycocalyx components heparan sulphate and syndecan-1, respectively [64, 66]. Cleavage of these important glycocalyx components and breakdown of intercellular junctions contributes to vascular leakage (Fig. 3). Since glycocalyceal function includes prevention of platelet adhesion and leucocyte activation and adhesion, injury to the glycocalyx can cause a self-perpetuating cycle of inflammation and further endothelial injury.
Several studies have demonstrated that soluble markers of glycocalyx breakdown, such as syndecan-1, hyaluronan and heparan sulphate, are associated with sepsis presence, severity, and mortality [67,68,69].
Intravenous fluid therapy, a key component of sepsis resuscitation, may exacerbate glycocalyceal injury [70,71,72]. The hormone atrial natriuretic peptide, released from cardiac atria in response to mechanical stretch, has been proposed as an important mediator of glycocalyx shedding [72,73,74]. Alternatively, rapid infusion of intravenous fluid may cause direct endothelial shear stress which may promote the activity of glycocalyx-shedding metalloproteinases [75] or cause neutrophil activation which may result in neutrophil elastase-induced endothelial injury [76, 77].
Vascular leakage and tissue hypoxia
In normal health a functioning and highly selective endothelial barrier is crucial to the maintenance of microvascular homeostasis. The angiopoietin-Tie 2 pathway is a complex, multifaceted cascade which is commonly implicated in vascular permeability. Tie 2 is a transmembrane endothelial tyrosine kinase [78]. Angiopoietin-1 (Ang-1) acts as a Tie 2 agonist and exerts a protective effect on the endothelium by promoting endothelial barrier function [79]. Ang-1, via Akt activation, inhibits the activity of the forkhead transcription factor which is a key regulator of genes associated with endothelial destabilisation [80]. In contrast, Angiopoietin-2 (Ang-2) is a context-dependent Tie 2 agonist or antagonist. The release of Ang-2 from Weibel-Palade bodies can be stimulated by key pro-permeability mediators such as thrombin and histamine [81]. In a murine LPS-induced endotoxaemic model of sepsis, Ang-2 binding resulted in Tie 2 antagonism [82], thus negating the protective effects of Ang-1 (Fig. 4). Moreover, Ang-2 binding to Tie 2 precipitates integrin degradation and endothelial barrier destabilisation [83]. In addition to Tie-2 antagonism, Ang-2 has been revealed to directly activate β1-integrin which resulted in cytoskeleton reorganisation and destabilization of intercellular junctions via increased cell contractility [84]. Thamm and colleagues have demonstrated increased Tie-2 cleavage in endothelial cells exposed to TNF- α, septic mice and septic humans [85]. Moreover, it was demonstrated that the matrix metalloprotease, MMP14, performed a central role in the cleavage of Tie-2 [85]. Furthermore, in a cecal ligation and puncture (CLP) model the investigators also demonstrated that Tie 2 transcription was dependent on flow [85, 86]. Absent flow, such as that observed in the septic microcirculation, was associated with reduced levels of GATA3, a flow dependent transcription factor which performs a key role in regulating Tie 2 transcription [85, 86].
In sepsis Ang-2 acts as an antagonist of Tie 2 which results in disruption of protective Ang-1/Tie 2 signalling. The antagonistic effects of Ang-2 leads to increased inflammation and inhibition of the vascular stabilising Akt signalling pathway. Moreover, the vascular barrier protective effects of Tie 2 are abrogated by the cleaving properties of MMP14
Importantly, Ang-2 has been identified as a prognostic biomarker in sepsis [87, 88], with Ang 2 levels correlating with disease severity and survival [89].The prominent role of the angiopoietin-Tie 2 pathway in endothelial dysfunction makes modulation of the Ang-1/Ang-2/Tie-2 equilibrium an attractive therapeutic target in sepsis. In a CLP model of sepsis, the use of a synthetic Tie 2 agonist was associated with an attenuated cytokine response, reduced vascular leakage, and improved organ function [90]. In another CLP model the use of Ang-2 small interfering RNA was associated with reduced IL-6 transcription and reduced levels of neutrophil infiltration, vascular leakage, and organ dysfunction [91].
Oxygen delivery occurs via diffusion of oxygen from capillary red blood cells to the mitochondria of tissue cells. Diffusion is dependent on the PO2 diffusion gradient between capillaries and tissue cells and on the diffusion distance from capillary red blood cells to tissue cell mitochondria [92]. In sepsis, heterogenous generation of NO, secondary to endothelial dysfunction, results in pathological vasodilatation and shunt formation with ensuant variable perfusion of tissue regions and cellular hypoxia in areas distant from perfused capillaries [92, 93]. Injury to the endothelial glycocalyx and to inter-endothelial junctional structures, culminating in interstitial oedema, may compound this problem as it increases diffusion distance between capillaries and cells (Fig. 5) [92]. Mechanical extrinsic compression of capillaries and lymphatics by interstitial fluid may further worsen oxygen delivery (Fig. 5) [94]. Exacerbation of tissue hypoxia by oedema may explain adverse outcomes associated with fluid overload in patients with sepsis [95,96,97].
Normal oxygen diffusion from blood vessels to target tissue cells. b Tissue hypoxia occurring due to increased diffusion distance between oxygen carrying red blood cells in the microvascular blood vessels and the mitochondria of tissue cells. c Tissue hypoxia occurring due to a tamponade like effect of interstitial fluid on microvascular blood vessels
Potential therapeutic approaches
Given the prominent role of endothelial breakdown and dysfunction in sepsis, preservation and restoration of endothelial function represents a key therapeutic target.
Imatinib and other tyrosine kinase inhibitors
The Abelson (Abl) family of tyrosine kinases, Abl (Abl1) and Arg (Abl2), perform an important role in cytoskeletal remodelling, adhesion, and migration [98]. Zandy et al., demonstrated the importance of Abl kinases in the formation and maintenance of adherens junctions [99]. The inhibition of tyrosine kinase Arg, also known as Abl2, serves to maintain endothelial barrier integrity. It has been demonstrated that depletion of Arg in endothelial cells is associated with reduced adherens junctions disruption and intercellular gap formation [100].
Imatinib, the most widely-studied Tyrosine Kinase Inhibitor, potentiates the activity of Rac 1 [101, 102], an endothelial barrier-supporting GTPase known to reinforce cell–matrix [103] and cell–cell interactions [104]. Imatinib targets the Abl family of non-receptor tyrosine kinases in addition to other tyrosine kinases, such as platelet-derived growth factor receptor, and the receptor tyrosine kinase Kit [105].
Vascular barrier protective effects of Imatinib have been identified in in vivo models of microcirculatory dysfunction and in patients with endothelial barrier disruption [106, 107]. In addition, the in vivo protective effects of Imatinib may be attributable to the effect on immune cells with Imatinib attenuating inflammation in animal models of LPS-induced lung injury [108, 109]. A potential clinical benefit has been demonstrated in patients with COVID-19, which shares many mechanistic features with sepsis [110]. There is, therefore, a growing body of evidence to support a potential role for the short-term administration of Imatinib as a therapeutic agent to maintain endothelial barrier integrity and attenuate inflammation in sepsis.
Selepressin
Vasopressin deficiency contributes to vascular dysfunction in septic shock [111] which provides the rationale for investigation of vasopressin receptor agonists in patients with sepsis. To date, however, vasopressin has failed to demonstrate clinical benefit over noradrenaline in sepsis [112]. One possible explanation is that non-specific vasopressin receptor stimulation can result in detrimental microcirculatory effects. Stimulation of endothelial V2 receptors can result in vasodilation via endothelial NOS activation [113], leucocyte adhesion and migration [114], secretion of procoagulant mediators [115] and salt and water retention [116].
Selepressin is a selective vasopressin V1a receptor agonist. In an ovine model of sepsis, animals receiving selepressin therapy had reduced vascular leakage compared to non-specific vasopressin receptor agonists and controls [117]. Moreover, selepressin therapy was associated with reduced myocardial and pulmonary tissue concentrations of VEGF and Ang-2 [117]. VEGF, a potent stimulator of vascular leakage, has been shown to increase the expression of Ang-2 in endothelial cells [118]. In sepsis, Ang-2 disrupts protective Tie2 signalling and contributes to endothelial barrier destabilisation [83]. However, despite the promising pre-clinical evidence base, in an RCT of 868 adult patients with septic shock receiving noradrenaline therapy, the use of selepressin did not improve clinical outcomes [119].
Mesenchymal stromal cells
Mesenchymal stromal cells (MSCs) are pluripotent stem cells that can differentiate into multiple cell types of mesenchymal lineage [120]. MSC treatment is associated with reduced organ dysfunction and coagulopathy in septic mice [121, 122]. Moreover, MSCs protect against LPS and VEGF induced barrier permeability in human umbilical vein endothelial cells (HUVECs) [122, 123]. Mechanistically, MSC treatment results in increased VE-cadherin levels and promotes VE-cadherin / beta-catenin interaction on endothelial cells [123]. The in vivo endothelial barrier protective effects of MSCs have been confirmed in a murine model of haemorrhagic shock where MSC administration resulted in reduced lung oedema and preservation of vascular tight junctions and adherens junctions [124].
A single centre pilot RCT which included 15 neutropenic patients with septic shock demonstrated more rapid haemodynamic stabilisation with prompt vasopressor weaning and improved PaO2/FiO2 ratios in those treated with MSC therapy [125]. Alp and colleagues subsequently confirmed the safety of MSCs in patients with sepsis and septic shock and identified reduced Sequential Organ Failure Assessment (SOFA) scores in patients receiving MSCs [126]. However, in a phase 1 dose escalation study in nine patients with septic shock, there was no efficacy signal in the MSC treatment arm [127].
The inflammatory-mediated barrier breakdown in ARDS overlaps with sepsis. Administration of mesenchymal stromal cell-derived extracellular vesicles (MSC-EVs) improves barrier integrity of human primary lung epithelial and endothelial cells following exposure to the plasma of patients with a hypoinflammatory ARDS phenotype [128]. Despite conflicting data on the effect of MSC therapy in phase 2 studies [129,130,131,132,133,134], there is evidence that MSCs have a protective effect on endothelium, providing a supportive rationale for further investigation in sepsis [134].
Statins
Statins possess an array of important pleiotropic effects [135, 136]. Zheng and colleagues identified that treatment of HUVECs with Simvastatin attenuated LPS-induced endothelial permeability by potentiating the activity of IQ‐GTPase‐activating protein 1, a regulator of cytoskeletal function [137]. Furthermore, in a rat model of endotoxaemia, Simvastatin treatment attenuated hepatic endothelial dysfunction and preserved the antithrombotic properties of sinusoidal endothelial cells disrupted by LPS [138, 139]. Statin therapy has also been shown to modify the activity of endothelial nitric oxide synthase, a key producer of NO, by preventing hypoxia and TNF- α induced downregulation [140]. In addition, it has been demonstrated that statin treatment prevents the nuclear translocation of NF-κB in endothelial cells subjected to pro-inflammatory stimuli [141].
A retrospective cohort analysis of hospitalised patients with bacteraemia identified a significant survival benefit in patients with pre-existing statin therapy [142] although this was not confirmed in a subsequent RCT by Kruger et al. [143]. However, continued statin therapy in patients with pre-existing use is associated with improved survival [143].
PCSK-9 inhibitors
Proprotein Convertase Subtilisin/Kexin-9 (PCSK-9) inhibitors are an emerging drug class with a growing evidence base for prevention of cardiovascular events in hypercholesterolaemia. PCSK-9 inhibitors inhibit the serine protease PCSK-9 and interfere with the LDL receptor recycling pathway, ultimately leading to recycling of the receptor and increased LDL cholesterol clearance [144]. PCSK-9 levels are elevated in patients with sepsis [145] and expression is upregulated in various models of sepsis [146]. Moreover, PCSK-9 knockout is associated with reduced bacterial dissemination, organ dysfunction and inflammation in a murine model of sepsis [147]. Importantly, PCSK-9 inhibition reverses impaired VE-cadherin expression observed in in vitro and in vivo models of sepsis [146].
Similar to statins, PCSK-9 inhibitors possess pluripotent properties. PSCK-9 deficient mice exhibit reduced expression of NADPH oxidase, a major source of ROS production which may further serve to protect the endothelium [148]. The anti-inflammatory effects of PCSK-9 inhibition have been demonstrated by Tang and colleagues who confirmed that in vitro PCSK-9 inhibition attenuates the generation of inflammatory cytokines by interfering with the NF-kB pathway [149].
In a placebo controlled, multicentre pilot trial, 60 patients with severe COVID-19 infection were randomised to receive 140-mg subcutaneous injection of Evolocumab, a PCSK-9 inhibitor, or placebo [150]. The investigators demonstrated that compared to placebo, PCSK-9 inhibition resulted in a greater reduction in IL-6 levels, a reduced requirement for invasive ventilation and improved mortality. This highlights the potential role of PCSK-9 inhibitors as endothelial barrier protective agents.
Alpha adrenoceptor agonists
Alpha adrenoceptor agonists such as Clonidine and Dexmedetomidine cause sympathetic inhibition and parasympathetic stimulation [151]. The expression of adrenergic receptors on endothelial cells provides a sound rationale for investigation of these agents.
Dexmedetomidine is a commonly used sedative which attenuates inflammatory cytokine production in septic patients [152]. In a LPS rat model of endotoxaemia, Dexmedetomidine administration was associated with attenuated TNF- α and IL-6 levels and a reduction in mortality [153]. This anti-inflammatory and mortality benefit has also been observed in CLP models of sepsis [154]. Moreover, Yeh and colleagues have demonstrated that Dexmedetomidine reduces tight junction damage, endothelial dysfunction, and microcirculatory impairment in endotoxaemic rats [155].
Similarly, there is a growing body of evidence to support the investigation of Clonidine in sepsis. In a CLP murine model of sepsis, the pre-emptive administration of Clonidine attenuated pro-inflammatory cytokine release, downregulated the binding activity of NF-κB, and reduced mortality [156]. Moreover, Schmidt and colleagues confirmed that Clonidine administration was effective in attenuating microvascular permeability in endotoxaemic rats [157].
Intermedin
Intermedin is a member of the calcitonin gene related peptide family which exerts its effects via the calcitonin receptor-like receptor signalling pathway [158]. Aslam and colleagues identified that Intermedin reduces HUVEC permeability and induces Rac1 activation, a key endothelial barrier supporting GTPase [159]. It has been determined that pre-treatment of mice with Intermedin attenuates vascular leakage in LPS and CLP models of sepsis [160]. Furthermore, the anti-inflammatory effects of Intermedin have been demonstrated in a CLP model of sepsis in which Intermedin tempered inflammatory cytokine production [160].
Adrenomedullin
Adrenomedullin (ADM), another member of the calcitonin gene related peptide family, is a vasoactive peptide hormone which regulates endothelial barrier function and vascular tone. It has been demonstrated that blood ADM levels correlate with vasopressor requirement and mortality in patients with sepsis [161, 162].
In vitro data has confirmed that ADM attenuates endothelial permeability in HUVECs [163, 164]. Moreover, in a Staphylococcus aureus toxin model of sepsis in rats, administration of ADM attenuated endothelial leakage and reduced mortality from 53 to 7% [165]. These outcomes suggest that ADM performs a key role in controlling endothelial barrier function and vascular tone, however, meticulous regulation is required. Of note, a multicentre phase 2 RCT investigating the safety and efficacy of inhaled pegylated adrenomedullin in adult patients with ARDS was recently stopped prematurely due to futility (NCT 04417036).
Adrecizumab
Adrecizumab is a non-neutralising ADM binding antibody which targets the N-terminus of ADM and only partially inhibits ADM signalling. In a CLP murine model of sepsis, Struck and colleagues demonstrated that the partial inhibition of ADM was more efficacious than an antibody which completely blocked ADM [166]. The investigators hypothesised that partial functional inhibition of ADM negates the harmful effects of excessive ADM while still preserving an adequate degree of ADM activity which may be required, especially in the early hyperdynamic phase of sepsis [166].
The endothelial barrier protective effects of Adrecizumab have been demonstrated in LPS and CLP rat models of inflammation and sepsis [167]. The AdrenOSS-2 trial, a biomarker-guided randomised trial, compared Adrecizumab with placebo in patients with septic shock and elevated concentrations of ADM. AdrenOSS-2 revealed that Adrecizumab was associated with a greater improvement in SOFA scores compared to placebo and demonstrated a trend towards decreased mortality (23.9% versus 27.7%) [168]. Although promising, further trials of Adrecizumab are needed.
Vitamin C
Vitamin C has been extensively investigated in the management of sepsis. Zhou et al., demonstrated that Vitamin C pre-treatment in a CLP model of sepsis reduced excessive production of NO and ROS and attenuated vascular leakage by preventing the dephosphorylation of occludin [169]. Occludin dephosphorylation results in disassembly of tight junctions and increased vascular permeability [170]. Pre-clinical and clinical evidence has highlighted that Vitamin C attenuates endotoxin induced lung injury [171] and oedema formation in patients with burn injuries [172]. However, despite a promising pre-clinical evidence base, results from RCTs investigating Vitamin C have been disappointing. A recent meta-analysis which included 37 trials concluded that parenteral Vitamin C therapy was not associated with a mortality benefit [173].
Canaglifozin
Canaglifozin is a sodium glucose co-transporter 2 inhibitor utilised in the management of diabetes mellitus. Canaglifozin is an activator of AMPK, a serine/threonine protein kinase, which exerts a protective effect on endothelial adherens junctions and tight junctions [174, 175]. Moreover, it has been established that Canaglifozin attenuates LPS induced vascular leakage in mice [176]. No clinical trials of Canaglifozin have been undertaken in sepsis to date.
Humanin
Mitochondrial derived peptides, such as Humanin, possess key biological properties which make them an attractive therapeutic strategy in sepsis. Humanin has potent cytoprotective properties and has been demonstrated to protect endothelial cells from hyperglycaemia and oxidative stress [177, 178]. Humanin may mediate this protection via increased expression of Krüppel-like factor 2, an important transcriptional regulator of endothelial function [177].
Urban and colleagues have recently determined that a synthetic derivative of humanin, Colivelin, protects against endothelial injury and glycocalyx damage in a murine CLP model of sepsis [179]. It was highlighted that Colivelin activates AMPK which may be responsible for the vascular protective effects observed in the treatment group.
Fresh frozen plasma (FFP)
Fresh Frozen Plasma (FFP) is used to correct clotting factor deficiencies in bleeding patients and in coagulopathic patients at risk of bleeding [180]. Some studies have identified reduced mortality following FFP administration irrespective of correction of the underlying coagulopathy [181, 182]. Therefore, in addition to correcting coagulation factor deficiencies it has been postulated that FFP may possess endothelial protective properties. Straat and colleagues investigated the effects of FFP administration in non-bleeding critically ill patients, half of whom had sepsis [183]. FFP treatment was associated with reduced syndecan-1 and factor VIII levels, potentially reflecting attenuation of endothelial injury [183].
Activated protein C
Activated protein C (APC) is an endogenous protein generated from an inactive precursor, protein C, via the action of the Thrombin-Thrombomodulin complex [184]. APC possesses anti-coagulant and anti-inflammatory actions and has been demonstrated to inhibit neutrophil chemotaxis [185] and prevent endothelial cell apoptosis [186]. These properties made APC an attractive therapeutic option to investigate in sepsis.
Feistritzer and Riewald identified that the thrombin induced hyperpermeability of HUVECs was attenuated with APC pre-treatment [187]. However, the in vivo effects of APC on endothelial permeability are conflicting [188, 189].
The first phase 3 study to investigate APC in sepsis included 1690 patients with severe sepsis [190], and reported significantly reduced mortality at 28-days (30.8% in the placebo group vs 24.7% in the APC group) [190], following which APC received marketing authorisation and approvals in patients with severe sepsis who were considered at high risk of mortality. However, subsequent trials failed to confirm these results [191, 192], and worldwide withdrawal of APC from the market followed.
In a recent secondary analysis of the PROWESS-SHOCK trial, Sinha and colleagues tested for heterogeneity of treatment effect in inflammatory phenotypes [193]. The investigators revealed that APC treatment was associated with a higher 28-day mortality in the hypoinflammatory phenotype (APC 24.3% vs Placebo 19.5%), whereas mortality was reduced in the hyperinflammatory phenotype (APC 33.0% vs Placebo 41.3%) [193]. APC may have been a victim of the phenotypic heterogeneity which besets sepsis.
Conclusion
Microvascular dysfunction is strongly associated with morbidity and mortality in sepsis. Microcirculatory dysfunction encompasses distinct pathological processes such as abnormal NO expression with ensuant heterogenous capillary perfusion, increased endothelial adhesiveness to leucocytes and platelets, dysregulation of smooth muscle cells with a loss of adrenergic sensitivity and increased endothelial permeability. This review has focused on the endothelial permeability aspect of microcirculatory dysfunction.
Despite the detrimental effects of vascular dysfunction and endothelial breakdown, no pharmacological therapies are currently used to attenuate vascular leakage. When putative endothelial protective agents have been studied in RCTs to date the results have been disappointing despite promising pre-clinical evidence. Future investigation of these agents should involve targeted treatment of endothelial injury in mechanistically orientated trials with a homogenous patient population. Ideally, this would involve development of diagnostic methods to facilitate rapid diagnosis and phenotyping of endothelial dysfunction and would provide a platform for observation of response to treatment alongside patient-centred clinical outcomes.
Acknowldgements
DM reports grants from NIHR, Innovate UK, MRC, Northern Ireland HSC R&D division, Wellcome Trust, Randox and Novavax as an investigator in ARDS and COVID-19 studies. DM reports consultancy fees unrelated to this work from Bayer, GlaxoSmithKline, Aptarion, Direct Biologics, Aviceda Boehringer Ingelheim, Novartis SOBU, and Eli Lilly. DM reports payments from GlaxoSmithKline as an educational seminar speaker. DM reports fees as a member of the DSMB for Vir Biotechnology, Inc and Faron Pharmaceuticals. DM has a patent for a novel treatment for inflammatory disease. DM was a Director of Research for the Intensive Care Society, Director of the MRC/NIHR EME programme and NIHR Scientific Director for Programmes. DM reports his spouse has received consultancy fees from INSMED and from the California Institute for Regenerative unrelated to this work. CO reports grants from the Wellcome Trust, MRC, NI HSC R&D Division, Innovate UK as an investigator in ARDS and COVID studies. CO reports consultancy from INSMED unrelated to this work and fees for grant panel membership from the Californian Institute of Regenerative Medicine. CO spouse reports (a) consultancy fees unrelated to this work from Bayer, GlaxoSmithKline, Aptarion, Direct Biologics, Aviceda Boehringer Ingelheim, Novartis SOBU, and Eli Lilly (b) payments from GlaxoSmithKline as an educational seminar speaker (c) fee as a member of the DSMB for Vir Biotechnology, Inc and Faron Pharmaceuticals(d) a patent for a novel treatment for inflammatory disease. CO spouse was a Director of Research for the Intensive Care Society, Director of the MRC/NIHR EME programme and NIHR Scientific Director for Programmes. JS reports grants from MRC, NI HSC R&D Division, NIAA and NIHR related to studies in sepsis. JS has received consultancy fees or honoraria unrelated to this work from Edwards Lifesciences, Baxter Healthcare, and Merck Sharpe Dolme, and research support from CASMED (now Edwards Lifesciences).
Availability of data and materials
Not Applicable.
Abbreviations
- Abl:
-
Abelson
- ADM:
-
Adrenomedullin
- Ang-1:
-
Angiopoietin-1
- Ang-2:
-
Angiopoietin-2
- APC:
-
Activated protein C
- ARDS:
-
Acute respiratory distress syndrome
- CLP:
-
Cecal ligation and puncture
- HUVECs:
-
Human umbilical vein endothelial cells
- IL:
-
Interleukin
- LPS:
-
Lipopolysaccharide
- MSCs:
-
Mesenchymal stromal cells
- MSC-EVs:
-
Mesenchymal stromal cell-derived extracellular vesicles
- NF-κB:
-
Nuclear factor-κB
- NO:
-
Nitric oxide
- PCSK-9:
-
Proprotein Convertase Subtilisin/Kexin-9
- RCT:
-
Randomised control trial
- ROS:
-
Reactive oxygen species
- SOFA:
-
Sequential Organ Failure Assessment
- TNF-α:
-
Tumour necrosis factor alpha
- VE-cadherin:
-
Vascular-endothelial cadherin
- VEGF:
-
Vascular endothelial growth factor
- ZO:
-
Zonula occludens
References
Singer M, Deutschman CS, Seymour CW, Shankar-Hari M, Annane D, Bauer M, et al. The third international consensus definitions for sepsis and septic shock (Sepsis-3). JAMA. 2016;315(8):801–10.
Organization WH. Global report on the epidemiology and burden of sepsis: current evidence, identifying gaps and future directions. 2020.
Bateman RM, Sharpe MD, Jagger JE, Ellis CG. Sepsis impairs microvascular autoregulation and delays capillary response within hypoxic capillaries. Crit Care. 2015;19(1):1–14.
Colbert JF, Schmidt EP. Endothelial and microcirculatory function and dysfunction in sepsis. Clin Chest Med. 2016;37(2):263–75.
Joffre J, Hellman J, Ince C, Ait-Oufella H. Endothelial responses in sepsis. Am J Respir Crit Care Med. 2020;202(3):361–70.
Ince C, Mayeux PR, Nguyen T, Gomez H, Kellum JA, Ospina-Tascón GA, et al. The endothelium in sepsis. Shock. 2016;45(3):259.
Fernández-Sarmiento J, Schlapbach LJ, Acevedo L, Santana CR, Acosta Y, Diana A, Monsalve MC, Carcillo JA. Endothelial damage in sepsis: the importance of systems biology. Front Pediatr. 2022;9(10):828968.
Krüger-Genge A, Blocki A, Franke RP, Jung F. Vascular endothelial cell biology: an update. Int J Mol Sci. 2019;20(18):4411.
Rajala R. How big is the endothelium? Comment on “spatial and temporal dynamics of the endothelium.” J Thromb Haemost. 2021;19(10):2634–5.
Aird WC. Spatial and temporal dynamics of the endothelium. J Thromb Haemost. 2005;3(7):1392–406.
Furchgott RF, Zawadzki JV. The obligatory role of endothelial cells in the relaxation of arterial smooth muscle by acetylcholine. Nature. 1980;288(5789):373–6.
Corson MA, James NL, Latta SE, Nerem RM, Berk BC, Harrison DG. Phosphorylation of endothelial nitric oxide synthase in response to fluid shear stress. Circ Res. 1996;79(5):984–91.
Govers R, Rabelink TJ. Cellular regulation of endothelial nitric oxide synthase. Am J Physiol Renal Physiol. 2001;280(2):F193-206.
López A, Lorente JA, Steingrub J, Bakker J, McLuckie A, Willatts S, et al. Multiple-center, randomized, placebo-controlled, double-blind study of the nitric oxide synthase inhibitor 546C88: effect on survival in patients with septic shock. Crit Care Med. 2004;32(1):21–30.
Vincent JL, Privalle CT, Singer M, Lorente JA, Boehm E, Meier-Hellmann A, et al. Multicenter, randomized, placebo-controlled phase III study of pyridoxalated hemoglobin polyoxyethylene in distributive shock (PHOENIX). Crit Care Med. 2015;43(1):57–64.
Boerma EC, Koopmans M, Konijn A, Kaiferova K, Bakker AJ, van Roon EN, et al. Effects of nitroglycerin on sublingual microcirculatory blood flow in patients with severe sepsis/septic shock after a strict resuscitation protocol: a double-blind randomized placebo controlled trial. Crit Care Med. 2010;38(1):93–100.
Trzeciak S, Glaspey LJ, Dellinger RP, Durflinger P, Anderson K, Dezfulian C, et al. Randomized controlled trial of inhaled nitric oxide for the treatment of microcirculatory dysfunction in patients with sepsis. Crit Care Med. 2014;42(12):2482–92.
Moncada S, Higgs EA, Vane JR. Human arterial and venous tissues generate prostacyclin (prostaglandin x), a potent inhibitor of platelet aggregation. The Lancet. 1977;309(8001):18–21.
Kinlay S, Behrendt D, Wainstein M, Beltrame J, Fang JC, Creager MA, et al. Role of endothelin-1 in the active constriction of human atherosclerotic coronary arteries. Circulation. 2001;104(10):1114–8.
Saye JA, Singer HA, Peach MJ. Role of endothelium in conversion of angiotensin I to angiotensin II in rabbit aorta. Hypertension. 1984;6(2_pt_1):216–21.
Bazzoni G, Dejana E. Endothelial cell-to-cell junctions: molecular organization and role in vascular homeostasis. Physiol Rev. 2004;84(3):869–901.
Kooij G, Van Horssen J, De Vries E. Tight junctions of the blood–brain barrier. Blood–Brain Barr Microenviron Basic Physiol Neurol Dis. 2005;38(6):47–69.
Risau W, Rubanyi GM. Structural, biochemical and functional differentiation of the vascular endothelium. Morphogenesis Endothel. 2000. https://doi.org/10.1201/9781482284119-2/structural-biochemical-functional-differentiation-vascular-endothelium-maya-simionescu.
Hartsock A, Nelson WJ. Adherens and tight junctions: structure, function and connections to the actin cytoskeleton. Biochim Biophys Acta BBA Biomembr. 2008;1778(3):660–9.
Carden D, Xiao F, Moak C, Willis BH, Robinson-Jackson S, Alexander S. Neutrophil elastase promotes lung microvascular injury and proteolysis of endothelial cadherins. Am J Physiol. 1998;275(2):385–92.
Schulz B, Pruessmeyer J, Maretzky T, Ludwig A, Blobel CP, Saftig P, et al. ADAM10 regulates endothelial permeability and T-cell transmigration by proteolysis of vascular endothelial cadherin. Circ Res. 2008;102(10):1192–201.
Hahmeyer ML, Da S, Da Silva-Santos JE. Rho-proteins and downstream pathways as potential targets in sepsis and septic shock: what have we learned from basic research. Cells. 2021;10(8):1844.
Mammoto T, Parikh SM, Mammoto A, Gallagher D, Chan B, Mostoslavsky G, et al. Angiopoietin-1 requires p190 RhoGAP to protect against vascular leakage in vivo. J Biol Chem. 2007;282(33):23910–8.
Xing J, Wang Q, Coughlan K, Viollet B, Moriasi C, Zou MH. Inhibition of AMP-activated protein kinase accentuates lipopolysaccharide-induced lung endothelial barrier dysfunction and lung injury in vivo. Am J Pathol. 2013;182(3):1021–30.
Haidari M, Zhang W, Chen Z, Ganjehei L, Mortazavi A, Warier N, et al. Atorvastatin preserves the integrity of endothelial adherens junctions by inhibiting vascular endothelial cadherin tyrosine phosphorylation. Exp Cell Res. 2012;318(14):1673–84.
Anderson JM, Van Itallie CM. Physiology and function of the tight junction. Cold Spring Harb Perspect Biol. 2009;1(2):a002584.
Zihni C, Mills C, Matter K, Balda MS. Tight junctions: from simple barriers to multifunctional molecular gates. Nat Rev Mol Cell Biol. 2016;17(9):564–80.
Matter K, Balda MS. Signalling to and from tight junctions. Nat Rev Mol Cell Biol. 2003;4(3):225–36.
Tornavaca O, Chia M, Dufton N, Almagro LO, Conway DE, Randi AM, et al. ZO-1 controls endothelial adherens junctions, cell–cell tension, angiogenesis, and barrier formation. J Cell Biol. 2015;208(6):821.
Hautefort A, Pfenniger A, Kwak BR. Endothelial connexins in vascular function. Vasc Biol. 2019;1(1):H117.
Clark PR, Kim RK, Pober JS, Kluger MS. Tumor necrosis factor disrupts claudin-5 endothelial tight junction barriers in two distinct NF-κB-dependent phases. PLoS ONE. 2015;10(3):e0120075.
Arnalich F, Garcia-Palomero E, López J, Jiménez M, Madero R, Renart J, et al. Predictive value of nuclear factor kappaB activity and plasma cytokine levels in patients with sepsis. Infect Immun. 2000;68(4):1942–5.
Chousterman BG, Swirski FK, Weber GF. Cytokine storm and sepsis disease pathogenesis. Semin Immunopathol. 2017;39(5):517–28.
Brooks D, Barr LC, Wiscombe S, McAuley DF, Simpson AJ, Rostron AJ. Human lipopolysaccharide models provide mechanistic and therapeutic insights into systemic and pulmonary inflammation. Eur Respir J. 2020;56(1):1901298.
Branger J, Knapp S, Weijer S, Leemans JC, Pater JM, Speelman P, et al. Role oftoll-like receptor 4 in gram-positive and gram-negative pneumonia inmice. Infect Immun. 2004;72(2):788–94.
Roger T, Froidevaux C, Le Roy D, Reymond MK, Chanson AL, Mauri D, et al. Protection from lethal gram-negative bacterial sepsis by targeting Toll-like receptor 4. Proc Natl Acad Sci. 2009;106(7):2348–52.
Kawai T, Akira S. The role of pattern-recognition receptors in innate immunity: update on Toll-like receptors. Nat Immunol. 2010;11(5):373–84.
Lim KH, Staudt LM. Toll-like receptor signaling. Cold Spring Harb Perspect Biol. 2013;5(1):a011247.
Tracey KJ, Beutler B, Lowry SF, Merryweather J, Wolpe S, Milsark IW, et al. Shock and tissue injury induced by recombinant human cachectin. Science. 1979;234(4775):470–4.
Tracey KJ, Fong Y, Hesse DG, Manogue KR, Lee AT, Kuo GC, et al. Anti-cachectin/TNF monoclonal antibodies prevent septic shock during lethal bacteraemia. Nature. 1987;330(6149):662–4.
Abraham E, Wunderink R, Silverman H, Perl TM, Nasraway S, Levy H, et al. Efficacy and safety of monoclonal antibody to human tumor necrosis factor α in patients with sepsis syndrome: a randomized, controlled, double-blind. Multicent Clin Trial JAMA. 1995;273(12):934–41.
Fisher CJ Jr, Agosti JM, Opal SM, Lowry SF, Balk RA, Sadoff JC, Abraham E, Schein RM, Benjamin E. Treatment of septic shock with the tumor necrosis factor receptor: Fc fusion protein. N Engl J Med. 1996;334(26):1697–702.
Melter M, Reinders MEJ, Sho M, Pal S, Geehan C, Denton MD, et al. Ligation of CD40 induces the expression of vascular endothelial growth factor by endothelial cells and monocytes and promotes angiogenesis in vivo. Blood. 2000;96(12):3801–8.
Shweiki D, Itin A, Soffer D, Keshet E. Vascular endothelial growth factor induced by hypoxia may mediate hypoxia-initiated angiogenesis. Nature. 1992;359(6398):843–5.
Ben-Av P, Crofford LJ, Wilder RL, Hla T. Induction of vascular endothelial growth factor expression in synovial fibroblasts by prostaglandin E and interleukin-1: a potential mechanism for inflammatory angiogenesis. FEBS Lett. 1995;372(1):83–7.
Hellwig-Bürgel T, Rutkowski K, Metzen E, Fandrey J, Jelkmann W. Interleukin-1β and tumor necrosis factor-α stimulate DNA binding of hypoxia-inducible factor-1. Blood. 1999;94(5):1561–7.
Roberts WG, Palade GE. Increased microvascular permeability and endothelial fenestration induced by vascular endothelial growth factor. J Cell Sci. 1995;108(6):2369–79.
Satchell SC, Braet F. Glomerular endothelial cell fenestrations: an integral component of the glomerular filtration barrier. Am J Physiol Renal Physiol. 2009;296(5):947–56.
Feng D, Nagy JA, Hipp J, Dvorak HF, Dvorak AM. Vesiculo–vacuolar organelles and the regulation of venule permeability to macromolecules by vascular permeability factor, histamine, and serotonin. J Exp Med. 1996;183(5):1981–6.
Feng Y, Venema V, Venema RC, Tsai N, Bebzadian MA, Caldwell RB. VEGF-induced permeability increase is mediated by caveolae. Invest Ophthalmol Vis Sci. 1999;40(1):157–67.
Kevil CG, Keith Payne D, Mire E, Alexander JS. Vascular permeability factor/vascular endothelial cell growth factor-mediated permeability occurs through disorganization of endothelial junctional proteins. J Biol Chem. 1998;273(24):15099–103.
Reitsma S, Slaaf DW, Vink H, Van Zandvoort MAMJ, Oude Egbrink MGA. The endothelial glycocalyx: composition, functions, and visualization. Pflugers Archiv. 2007;454(3):345.
Rehm M, Zahler S, Lötsch M, Welsch U, Conzen P, Jacob M, et al. Endothelial glycocalyx as an additional barrier determining extravasation of 6% hydroxyethyl starch or 5% albumin solutions in the coronary vascular bed. J Am Soc Anesthesiol. 2004;100(5):1211–23.
Fernández-Sarmiento J, Salazar-Peláez LM, Carcillo JA. The endothelial glycocalyx: a fundamental determinant of vascular permeability in sepsis. Pediatr Crit Care Med. 2020;21(5):e291.
Woodcock TE, Woodcock TM. Revised starling equation and the glycocalyx model of transvascular fluid exchange: an improved paradigm for prescribing intravenous fluid therapy. Br J Anaesth. 2012;108(3):384–94.
Uchimido R, Schmidt EP, Shapiro NI. The glycocalyx: a novel diagnostic and therapeutic target in sepsis. Crit Care. 2019;23(1):1–12.
Iba T, Levy JH. Derangement of the endothelial glycocalyx in sepsis. J Thromb Haemost. 2019;17(2):283–94.
Moore KH, Murphy HA, George EM. The glycocalyx: a central regulator of vascular function. Am J Physiol Regul Integr Comparative Physiol. 2021;320(4):R508–18.
Schmidt EP, Yang Y, Janssen WJ, Gandjeva A, Perez MJ, Barthel L, et al. The pulmonary endothelial glycocalyx regulates neutrophil adhesion and lung injury during experimental sepsis. Nat Med. 2012;18(8):1217–23.
Lipowsky HH, Lescanic A. The effect of doxycycline on shedding of the glycocalyx due to reactive oxygen species. Microvasc Res. 2013;90:80–5.
Manon-Jensen T, Multhaupt HAB, Couchman JR. Mapping of matrix metalloproteinase cleavage sites on syndecan-1 and syndecan-4 ectodomains. FEBS J. 2013;280(10):2320–31.
Anand D, Ray S, Srivastava LM, Bhargava S. Evolution of serum hyaluronan and syndecan levels in prognosis of sepsis patients. Clin Biochem. 2016;49(10–11):768–76.
Yagmur E, Koch A, Haumann M, Kramann R, Trautwein C, Tacke F. Hyaluronan serum concentrations are elevated in critically ill patients and associated with disease severity. Clin Biochem. 2012;45(1–2):82–7.
Nelson A, Berkestedt I, Bodelsson M. Circulating glycosaminoglycan species in septic shock. Acta Anaesthesiol Scand. 2014;58(1):36–43.
Hippensteel JA, Uchimido R, Tyler PD, Burke RC, Han X, Zhang F, et al. Intravenous fluid resuscitation is associated with septic endothelial glycocalyx degradation. Crit Care. 2019;23(1):1–10.
Byrne L, Obonyo NG, Diab SD, Dunster KR, Passmore MR, Boon AC, et al. Unintended consequences: fluid resuscitation worsens shock in an ovine model of endotoxemia. Am J Respir Crit Care Med. 2018;198(8):1043–54.
Chappell D, Bruegger D, Potzel J, Jacob M, Brettner F, Vogeser M, et al. Hypervolemia increases release of atrial natriuretic peptide and shedding of the endothelial glycocalyx. Crit Care. 2014;18(5):1–8.
Bruegger D, Jacob M, Rehm M, Loetsch M, Welsch U, Conzen P, et al. Atrial natriuretic peptide induces shedding of endothelial glycocalyx in coronary vascular bed of guinea pig hearts. Am J Physiol Heart Circ Physiol. 2005;289(5):1993.
Jacob M, Saller T, Chappell D, Rehm M, Welsch U, Becker BF. Physiological levels of A-, B- and C-type natriuretic peptide shed the endothelial glycocalyx and enhance vascular permeability. Basic Res Cardiol. 2013;108(3):1–9.
Kang H, Duran CL, Abbey CA, Kaunas RR, Bayless KJ. Fluid shear stress promotes proprotein convertase-dependent activation of MT1-MMP. Biochem Biophys Res Commun. 2015;460(3):596–602.
Rhee P, Wang D, Ruff P, Austin B, DeBraux S, Wolcott K, et al. Human neutrophil activation and increased adhesion by various resuscitation fluids. Crit Care Med. 2000;28(1):74–8.
Suzuki K, Okada H, Takemura G, Takada C, Kuroda A, Yano H, et al. Neutrophil elastase damages the pulmonary endothelial glycocalyx in lipopolysaccharide-induced experimental endotoxemia. Am J Pathol. 2019;189(8):1526–35.
van der Heijden M, van Nieuw Amerongen GP, Chedamni S, van Hinsbergh VWM, Johan Groeneveld AB. The angiopoietin-Tie2 system as a therapeutic target in sepsis and acute lung injury. Expert Opin Ther Targets. 2009;13(1):39–53.
Koh GY. Orchestral actions of angiopoietin-1 in vascular regeneration. Trends Mol Med. 2013;19(1):31–9.
Daly C, Wong V, Burova E, Wei Y, Zabski S, Griffiths J, et al. Angiopoietin-1 modulates endothelial cell function and gene expression via the transcription factor FKHR (FOXO1). Genes Dev. 2004;18(9):1060–71.
Fiedler U, Scharpfenecker M, Koidl S, Hegen A, Grunow V, Schmidt JM, et al. The Tie-2 ligand angiopoietin-2 is stored in and rapidly released upon stimulation from endothelial cell Weibel-Palade bodies. Blood. 2004;103(11):4150–6.
Korhonen EA, Lampinen A, Giri H, Anisimov A, Kim M, Allen B, et al. Tie1 controls angiopoietin function in vascular remodeling and inflammation. J Clin Invest. 2016;126(9):3495–510.
Thomas M, Felcht M, Kruse K, Kretschmer S, Deppermann C, Biesdorf A, et al. Angiopoietin-2 stimulation of endothelial cells induces αvβ3 integrin internalization and degradation. J Biol Chem. 2010;285(31):23842–9.
Hakanpaa L, Sipila T, Leppanen VM, Gautam P, Nurmi H, Jacquemet G, Eklund L, Ivaska J, Alitalo K, Saharinen P. Endothelial destabilization by angiopoietin-2 via integrin β1 activation. Nat Commun. 2015;6(1):5962.
Thamm K, Schrimpf C, Retzlaff J, Idowu TO, van Meurs M, Zijlstra JG, Ghosh CC, Zeitvogel J, Werfel TA, Haller H, Parikh SM. Molecular regulation of acute Tie2 suppression in sepsis. Critic Care Med. 2018;46(9):e928–36.
Idowu TO, Etzrodt V, Pape T, Heineke J, Stahl K, Haller H, et al. Flow-dependent regulation of endothelial Tie2 by GATA3 in vivo. Intensive Care Med Exp. 2021;9(1):1–14.
Orfanos SE, Kotanidou A, Glynos C, Athanasiou C, Tsigkos S, Dimopoulou I, et al. Angiopoietin-2 is increased in severe sepsis: correlation with inflammatory mediators. Crit Care Med. 2007;35(1):199–206.
Fang Y, Li C, Shao R, Yu H, Zhang Q, Zhao L. Prognostic significance of the angiopoietin-2/angiopoietin-1 and angiopoietin-1/Tie-2 ratios for early sepsis in an emergency department. Crit Care. 2015;19(1):1–11.
David S, Mukherjee A, Ghosh CC, Yano M, Khankin EV, Wenger JB, et al. Angiopoietin-2 may contribute to multi-organ dysfunction and death in sepsis. Crit Care Med. 2012;40(11):3034.
Kümpers P, Gueler F, David S, Van Slyke P, Dumont DJ, Park JK, et al. The synthetic tie2 agonist peptide vasculotide protects against vascular leakage and reduces mortality in murine abdominal sepsis. Crit Care. 2011;15(5):1–4.
Stiehl T, Thamm K, Kaufmann J, Schaeper U, Kirsch T, Haller H, et al. Lung-targeted RNA interference against angiopoietin-2 ameliorates multiple organ dysfunction and death in sepsis. Crit Care Med. 2014;42(10):e654–62.
Mallat J, Rahman N, Hamed F, Hernandez G, Fischer MO. Pathophysiology, mechanisms, and managements of tissue hypoxia. Anaesth Crit Care Pain Med. 2022;41(4):101087.
Pool R, Gomez H, Kellum JA. Mechanisms of organ dysfunction in sepsis. Crit Care Clin. 2018;34(1):63–80.
Ince C. Hemodynamic coherence and the rationale for monitoring the microcirculation. Crit Care. 2015;19(3):1–13.
Tigabu BM, Davari M, Kebriaeezadeh A, Mojtahedzadeh M. Fluid volume, fluid balance and patient outcome in severe sepsis and septic shock: a systematic review. J Crit Care. 2018;48:153–9.
Boyd JH, Forbes J, Nakada T, Walley KR, Russell JA. Fluid resuscitation in septic shock: a positive fluid balance and elevated central venous pressure are associated with increased mortality. Crit Care Med. 2011;39(2):259–65.
Sadaka F, Juarez M, Naydenov S, O’Brien J. Fluid resuscitation in septic shock: the effect of increasing fluid balance on mortality. J Intensive Care Med. 2014;29(4):213–7.
Greuber EK, Smith-Pearson P, Wang J, Pendergast AM. Role of ABL family kinases in cancer: from leukaemia to solid tumours. Nat Rev Cancer. 2013;13(8):559–71.
Zandy NL, Playford M, Pendergast AM. Abl tyrosine kinases regulate cell–cell adhesion through Rho GTPases. Proc Natl Acad Sci. 2007;104(45):17686–91.
Amado-Azevedo J, van Stalborch AMD, Valent ET, Nawaz K, van Bezu J, Eringa EC, et al. Depletion of Arg/Abl2 improves endothelial cell adhesion and prevents vascular leak during inflammation. Angiogenesis. 2021;24(3):677–93.
Chislock EM, Pendergast AM. Abl family kinases regulate endothelial barrier function in vitro and in mice. PLoS ONE. 2013;8(12):e85231.
Aman J, van Bezu J, Damanafshan A, Huveneers S, Eringa EC, Vogel SM, et al. Effective treatment of edema and endothelial barrier dysfunction with imatinib. Circulation. 2012;126(23):2728–38.
Birukova AA, Alekseeva E, Cokic I, Turner CE, Birukov KG. Cross talk between paxillin and Rac is critical for mediation of barrier-protective effects by oxidized phospholipids. Am J Physiol Lung Cell Mol Physiol. 2008;295(4):L593-602.
Mehta D, Malik AB. Signaling mechanisms regulating endothelial permeability. Physiol Rev. 2006;86(1):279–367.
Siehl J, Thiel E. C-kit, GIST, and imatinib. Target Ther Cancer. 2007;1:145–51.
Koning NJ, de Lange F, van Meurs M, Jongman RM, Ahmed Y, Schwarte LA, et al. Reduction of vascular leakage by imatinib is associated with preserved microcirculatory perfusion and reduced renal injury markers in a rat model of cardiopulmonary bypass. Br J Anaesth. 2018;120(6):1165–75.
Aman J, Peters MJL, Weenink C, van Nieuw Amerongen GP, Vonk NA. Reversal of vascular leak with imatinib. Am J Respir Crit Care Med. 2013;188(9):1171–3.
Stephens RS, Johnston L, Servinsky L, Kim BS, Damarla M. The tyrosine kinase inhibitor imatinib prevents lung injury and death after intravenous LPS in mice. Physiol Rep. 2015;3(11):e12589.
Rizzo AN, Aman J, van Nieuw Amerongen GP, Dudek SM. Targeting Abl kinases to regulate vascular leak during sepsis and acute respiratory distress syndrome. Arterioscler Thromb Vasc Biol. 2015;35(5):1071–9.
Aman J, Duijvelaar E, Botros L, Kianzad A, Schippers JR, Smeele PJ, Azhang S, Bartelink IH, Bayoumy AA, Bet PM, Boersma W. Imatinib in patients with severe COVID-19: a randomised, double-blind, placebo-controlled, clinical trial. Lancet Respir Med. 2021;9(9):957–68.
Landry DW, Levin HR, Gallant EM, Ashton RC, Seo S, D’Alessandro D, et al. Vasopressin deficiency contributes to the vasodilation of septic shock. Circulation. 1997;95(5):1122–5.
Russell JA, Walley KR, Singer J, Gordon AC, Hébert PC, Cooper DJ, et al. Vasopressin versus norepinephrine infusion in patients with septic shock. N Engl J Med. 2008;358(9):877–87.
Kaufmann JE, Iezzi M, Vischer UM. Desmopressin (DDAVP) induces NO production in human endothelial cells via V2 receptor-and cAMP-mediated signaling. J Thromb Haemost. 2003;1(4):821–8.
Kanwar S, Woodman RC, Poon MC, Murohara T, Lefer AM, Davenpeck KL, et al. Desmopressin induces endothelial P-selectin expression and leukocyte rolling in postcapillary venules. 1995;86(7):2760–6.
Rehberg S, Enkhbaatar P, Rehberg J, La E, Ferdyan N, Qi S, et al. Unlike arginine vasopressin, the selective V1a receptor agonist FE 202158 does not cause procoagulant effects by releasing von Willebrand factor. Crit Care Med. 2012;40(6):1957.
Kortenoeven MLA, Pedersen NB, Rosenbaek LL, Fenton RA. Vasopressin regulation of sodium transport in the distal nephron and collecting duct. Am J Physiol Renal Physiol. 2015;309(4):F280–99.
Rehberg S, Yamamoto Y, Sousse L, Bartha E, Jonkam C, Hasselbach AK, Traber LD, Cox RA, Westphal M, Enkhbaatar P, Traber DL. Selective V1a agonism attenuates vascular dysfunction and fluid accumulation in ovine severe sepsis. Am J Physiol Heart Circul Physiol. 2012;303(10):H1245–54.
Mandriota SJ, Pepper MS. Regulation of angiopoietin-2 mRNA levels in bovine microvascular endothelial cells by cytokines and hypoxia. Circ Res. 1998;83(8):852–9.
Laterre PF, Berry SM, Blemings A, Carlsen JE, François B, Graves T, et al. Effect of selepressin vs placebo on ventilator-and vasopressor-free days in patients with septic shock: the SEPSIS-ACT randomized clinical trial. JAMA. 2019;322(15):1476–85.
Friedenstein AJ, Petrakova KV, Kurolesova AI, Frolova GP. Heterotopic transplants of bone marrow. Transplantation. 1968;6(2):230–47.
Németh K, Leelahavanichkul A, Yuen PST, Mayer B, Parmelee A, Robey PG, et al. Bone marrow stromal cells attenuate sepsis via prostaglandin E2–dependent reprogramming of host macrophages to increase their interleukin-10 production. Nat Med. 2009;15(1):42–9.
Xu S, Zhou Z, Li H, Liu Z, Pan X, Wang F, et al. BMSCs ameliorate septic coagulopathy by suppressing inflammation in cecal ligation and puncture-induced sepsis. J Cell Sci. 2018;131(3):jcs211151.
Pati S, Khakoo AY, Zhao J, Jimenez F, Gerber MH, Harting M, et al. Human mesenchymal stem cells inhibit vascular permeability by modulating vascular endothelial cadherin/β-catenin signaling. Stem Cells Dev. 2011;20(1):89–101.
Pati S, Gerber MH, Menge TD, Wataha KA, Zhao Y, Baumgartner JA, et al. Bone marrow derived mesenchymal stem cells inhibit inflammation and preserve vascular endothelial integrity in the lungs after hemorrhagic shock. PLoS ONE. 2011;6(9):e25171.
Galstyan G, Makarova P, Parovichnikova E, Kuzmina L, Troitskaya V, Gemdzhian E, et al. The results of the single center pilot randomized Russian clinical trial of mesenchymal stromal cells in severe neutropenic patients with septic shock (RUMCESS). Int J Blood Res Disord. 2018;5(1):33.
Alp E, Gonen ZB, Gundogan K, Esmaoglu A, Kaynar L, Cetin A, et al. The effect of mesenchymal stromal cells on the mortality of patients with sepsis and septic shock: a promising therapy. Emerg Med Int. 2022;2022:9222379.
McIntyre LA, Stewart DJ, Mei SHJ, Courtman D, Watpool I, Granton J, et al. Cellular immunotherapy for septic shock. A phase I clinical trial. Am J Respir Crit Care Med. 2018;197(3):337–47.
Silva JD, Su Y, Calfee CS, Delucchi KL, Weiss D, McAuley DF, et al. Mesenchymal stromal cell extracellular vesicles rescue mitochondrial dysfunction and improve barrier integrity in clinically relevant models of ARDS. Eur Respir J. 2021;58(1):2002978.
Lanzoni G, Linetsky E, Correa D, Messinger Cayetano S, Alvarez RA, Kouroupis D, et al. Umbilical cord mesenchymal stem cells for COVID-19 acute respiratory distress syndrome: a double-blind, phase 1/2a, randomized controlled trial. Stem Cells Transl Med. 2021;10(5):660–73.
Gorman EA, Rynne J, Gardiner HJ, Rostron AJ, Bannard-Smith J, Bentley AM, Brealey D, Campbell C, Curley G, Clarke M, Dushianthan A. Repair of acute respiratory distress syndrome in COVID-19 by stromal cells (REALIST-COVID Trial): a multicenter, randomized, controlled clinical trial. Am J Respir Crit Care Med. 2023;208(3):256–69.
Bowdish ME, Barkauskas CE, Overbey JR, Gottlieb RL, Osman K, Duggal A, Marks ME, Hupf J, Fernandes E, Leshnower BG, Golob JL. A randomized trial of mesenchymal stromal cells for moderate to severe acute respiratory distress syndrome from COVID-19. Am J Respir Crit Care Med. 2023;207(3):261–70.
Kirkham AM, Bailey AJ, Monaghan M, Shorr R, Lalu MM, Fergusson DA, Allan DS. Updated living systematic review and meta-analysis of controlled trials of mesenchymal stromal cells to treat COVID-19: a framework for accelerated synthesis of trial evidence for rapid approval—FASTER approval. Stem Cells Transl Med. 2022;11(7):675–87.
Bellingan G, Jacono F, Bannard-Smith J, Brealey D, Meyer N, Thickett D, et al. Safety and efficacy of multipotent adult progenitor cells in acute respiratory distress syndrome (MUST-ARDS): a multicentre, randomised, double-blind, placebo-controlled phase 1/2 trial. Intensive Care Med. 2022;48:36–44.
Matthay MA, Calfee CS, Zhuo H, Thompson BT, Wilson JG, Levitt JE, Rogers AJ, Gotts JE, Wiener-Kronish JP, Bajwa EK, Donahoe MP. Treatment with allogeneic mesenchymal stromal cells for moderate to severe acute respiratory distress syndrome (START study): a randomised phase 2a safety trial. Lancet Respir Med. 2019;7(2):154–62.
Suda K, Eom J, Jaw J, Mui T, Bai N, Or C, et al. Endotoxin-induced cardiovascular dysfunction in mice: effect of simvastatin. J Appl Physiol. 2011;111(4):1118–24.
Giusti-Paiva A, Martinez MR, Felix JVC, da Rocha MJA, Carnio EC, Elias LLK, et al. Simvastatin decreases nitric oxide overproduction and reverts the impaired vascular responsiveness induced by endotoxic shock in rats. Shock. 2004;21(3):271–5.
Zheng X, Zhang W, Wang Z. Simvastatin preparations promote PDGF-BB secretion to repair LPS-induced endothelial injury through the PDGFRβ/PI3K/Akt/IQGAP1 signalling pathway. J Cell Mol Med. 2019;23(12):8314–27.
La Mura V, Pasarín M, Meireles CZ, Miquel R, Rodríguez-Vilarrupla A, Hide D, et al. Effects of simvastatin administration on rodents with lipopolysaccharide-induced liver microvascular dysfunction. Hepatology. 2013;57(3):1172–81.
La Mura V, Gagliano N, Arnaboldi F, Sartori P, Procacci P, Denti L, Liguori E, Bitto N, Ristagno G, Latini R, Dondossola D. Simvastatin prevents liver microthrombosis and sepsis induced coagulopathy in a rat model of endotoxemia. Cells. 2022;11(7):1148.
Laufs U, La Fata V, Liao JK. Inhibition of 3-hydroxy-3-methylglutaryl (HMG)-CoA reductase blocks hypoxia-mediated down-regulation of endothelial nitric oxide synthase. J Biol Chem. 1997;272(50):31725–9.
Hölschermann H, Schuster D, Parviz B, Haberbosch W, Tillmanns H, Muth H. Statins prevent NF-κB transactivation independently of the IKK-pathway in human endothelial cells. Atherosclerosis. 2006;185(2):240–5.
Kruger P, Fitzsimmons K, Cook D, Jones M, Nimmo G. Statin therapy is associated with fewer deaths in patients with bacteraemia. Intensive Care Med. 2006;32:75–9.
Kruger P, Bailey M, Bellomo R, Cooper DJ, Harward M, Higgins A, et al. A multicenter randomized trial of atorvastatin therapy in intensive care patients with severe sepsis. Am J Respir Crit Care Med. 2013;187(7):743–50.
Coppinger C, Movahed MR, Azemawah V, Peyton L, Gregory J, Hashemzadeh M. A comprehensive review of PCSK9 inhibitors. J Cardiovasc Pharmacol Ther. 2022;27:10742484221100108.
Innocenti F, Gori AM, Giusti B, Tozzi C, Donnini C, Meo F, et al. Plasma PCSK9 levels and sepsis severity: an early assessment in the emergency department. Clin Exp Med. 2021;21(1):101–7.
Huang L, Li Y, Cheng Z, Lv Z, Luo S, Xia Y. PCSK9 promotes endothelial dysfunction during sepsis via the TLR4/MyD88/NF-κB and NLRP3 pathways. Inflammation. 2023;46(1):115–28.
Dwivedi DJ, Grin PM, Khan M, Prat A, Zhou J, Fox-Robichaud AE, Seidah NG, Liaw PC. Differential expression of PCSK9 modulates infection, inflammation, and coagulation in a murine model of sepsis. Shock. 2016;46(6):672–80.
Ding Z, Liu S, Wang X, Deng X, Fan Y, Sun C, et al. Hemodynamic shear stress via ROS modulates PCSK9 expression in human vascular endothelial and smooth muscle cells and along the mouse aorta. Antioxid Redox Signal. 2015;22(9):760–71.
Tang ZH, Peng J, Ren Z, Yang J, Li TT, Li TH, et al. New role of PCSK9 in atherosclerotic inflammation promotion involving the TLR4/NF-κB pathway. Atherosclerosis. 2017;262:113–22.
Navarese EP, Podhajski P, Gurbel PA, Grzelakowska K, Ruscio E, Tantry U, et al. PCSK9 inhibition during the inflammatory stage of SARS-CoV-2 infection. J Am Coll Cardiol. 2023;81(3):224–34.
Toader E, Cividjian A, Rentero N, McAllen RM, Quintin L. Cardioinhibitory actions of clonidine assessed by cardiac vagal motoneuron recordings. J Hypertens. 2008;26(6):1169–80.
Memiş D, Hekimoğlu S, Vatan I, Yandım T, Yüksel M, Süt N. Effects of midazolam and dexmedetomidine on inflammatory responses and gastric intramucosal pH to sepsis, in critically ill patients. Br J Anaesth. 2007;98(4):550–2.
Taniguchi T, Kidani Y, Kanakura H, Takemoto Y, Yamamoto K. Effects of dexmedetomidine on mortality rate and inflammatory responses to endotoxin-induced shock in rats. Crit Care Med. 2004;32(6):1322–6.
Xu L, Bao H, Si Y, Wang X. Effects of dexmedetomidine on early and late cytokines during polymicrobial sepsis in mice. Inflamm Res. 2013;62(5):507–14.
Yeh YC, Wu CY, Cheng YJ, Liu CM, Hsiao JK, Chan WS, et al. Effects of dexmedetomidine on intestinal microcirculation and intestinal epithelial barrier in endotoxemic rats. Anesthesiology. 2016;125(2):355–67.
Hofer S, Steppan J, Wagner T, Funke B, Lichtenstern C, Martin E, et al. Central sympatholytics prolong survival in experimental sepsis. Crit Care. 2009;13(1):1–8.
Schmidt K, Hernekamp JF, Philipsenburg C, Zivkovic AR, Brenner T, Hofer S. Time-dependent effect of clonidine on microvascular permeability during endotoxemia. Microvasc Res. 2015;101:111–7.
Roh J, Chang CL, Bhalla A, Klein C, Hsu SYT. Intermedin is a calcitonin/calcitonin gene-related peptide family peptide acting through the calcitonin receptor-like receptor/receptor activity-modifying protein receptor complexes. J Biol Chem. 2004;279(8):7264–74.
Aslam M, Pfeil U, Gündüz D, Rafiq A, Kummer W, Piper HM, et al. Intermedin (adrenomedullin2) stabilizes the endothelial barrier and antagonizes thrombin-induced barrier failure in endothelial cell monolayers. Br J Pharmacol. 2012;165(1):208–22.
Xiao F, Wang D, Kong L, Li M, Feng Z, Shuai B, et al. Intermedin protects against sepsis by concurrently re-establishing the endothelial barrier and alleviating inflammatory responses. Nat Commun. 2018;9(1):1–15.
Marino R, Struck J, Maisel AS, Magrini L, Bergmann A, Di SS. Plasma adrenomedullin is associated with short-term mortality and vasopressor requirement in patients admitted with sepsis. Crit Care. 2014;18(1):1–7.
Guignant C, Voirin N, Venet F, Poitevin F, Malcus C, Bohé J, et al. Assessment of pro-vasopressin and pro-adrenomedullin as predictors of 28-day mortality in septic shock patients. Intensive Care Med. 2009;35(11):1859–67.
Hippenstiel S, Witzenrath M, Schmeck B, Hocke A, Krisp M, Krüll M, et al. Adrenomedullin reduces endothelial hyperpermeability. Circ Res. 2002;91(7):618–25.
Brell B, Temmesfeld-Wollbrück B, Altzschner I, Frisch E, Schmeck B, Hocke AC, et al. Adrenomedullin reduces Staphylococcus aureus α-toxin–induced rat ileum microcirculatory damage. Crit Care Med. 2005;33(4):819–26.
Temmesfeld-Wollbrück B, Brell B, Dávid I, Dorenberg M, Adolphs J, Schmeck B, et al. Adrenomedullin reduces vascular hyperpermeability and improves survival in rat septic shock. Intensive Care Med. 2007;33(4):703–10.
Struck J, Hein F, Karasch S, Bergmann A. Epitope specificity of anti-Adrenomedullin antibodies determines efficacy of mortality reduction in a cecal ligation and puncture mouse model. Intensive Care Med Exp. 2013;1(1):1–11.
Geven C, Peters E, Schroedter M, Struck J, Bergmann A, McCook O, et al. Effects of the humanized anti-adrenomedullin antibody adrecizumab (HAM8101) on vascular barrier function and survival in rodent models of systemic inflammation and sepsis. Shock. 2018;50(6):648–54.
Laterre PF, Pickkers P, Marx G, Wittebole X, Meziani F, Dugernier T, et al. Safety and tolerability of non-neutralizing adrenomedullin antibody adrecizumab (HAM8101) in septic shock patients: the AdrenOSS-2 phase 2a biomarker-guided trial. Intensive Care Med. 2021;47(11):1284–94.
Zhou G, Kamenos G, Pendem S, Wilson JX, Wu F. Ascorbate protects against vascular leakage in cecal ligation and puncture-induced septic peritonitis. Am J Physiol Regul Integr Comparative Physiol. 2012;302(4):R409–16.
Nunbhakdi-Craig V, Machleidt T, Ogris E, Bellotto D, White CL III, Sontag E. Protein phosphatase 2A associates with and regulates atypical PKC and the epithelial tight junction complex. J Cell Biol. 2002;158(5):967–78.
Dwenger A, Pape HC, Bantel C, Schweitzer G, Krumm K, Grotz M, et al. Ascorbic acid reduces the endotoxin-induced lung injury in awake sheep. Eur J Clin Invest. 1994;24(4):229–35.
Tanaka H, Matsuda T, Miyagantani Y, Yukioka T, Matsuda H, Shimazaki S. Reduction of resuscitation fluid volumes in severely burned patients using ascorbic acid administration: a randomized, prospective study. Arch Surg. 2000;135(3):326–31.
Agarwal A, Basmaji J, Fernando SM, Ge FZ, Xiao Y, Faisal H, et al. Parenteral vitamin C in patients with severe infection: a systematic review. NEJM Evidence. 2022;1(9):EVIDoa2200105.
Angé M, Castanares-Zapatero D, De Poortere J, Dufeys C, Courtoy GE, Bouzin C, et al. α1AMP-activated protein kinase protects against lipopolysaccharide-induced endothelial barrier disruption via junctional reinforcement and activation of the p38 MAPK/HSP27 pathway. Int J Mol Sci. 2020;21(15):5581.
Castanares-Zapatero D, Bouleti C, Sommereyns C, Gerber B, Lecut C, Mathivet T, et al. Connection between cardiac vascular permeability, myocardial edema, and inflammation during sepsis: role of the α: 1: AMP-activated protein kinase isoform. Crit Care Med. 2013;41(12):e411–22.
Angé M, De Poortere J, Ginion A, Battault S, Dechamps M, Muccioli GG, et al. Canagliflozin protects against sepsis capillary leak syndrome by activating endothelial α1AMPK. Sci Rep. 2021;11(1):1–13.
Wang X, Wu Z, He Y, Zhang H, Tian L, Zheng C, et al. Humanin prevents high glucose-induced monocyte adhesion to endothelial cells by targeting KLF2. Mol Immunol. 2018;101:245–50.
Bachar AR, Scheffer L, Schroeder AS, Nakamura HK, Cobb LJ, Oh YK, et al. Humanin is expressed in human vascular walls and has a cytoprotective effect against oxidized LDL-induced oxidative stress. Cardiovasc Res. 2010;88(2):360–6.
Urban C, Hayes HV, Piraino G, Wolfe V, Lahni P, O’Connor M, et al. Colivelin, a synthetic derivative of humanin, ameliorates endothelial injury and glycocalyx shedding after sepsis in mice. Front Immunol. 2022;13:984298.
Duguid J, O’Shaughnessy DF, Atterbury C, Maggs PB, Murphy M, Thomas D, et al. Guidelines for the use of fresh-frozen plasma, cryoprecipitate and cryosupernatant. Br J Haematol. 2004;126(1):11–28.
Chambers LA, Chow SJ, Shaffer LET. Frequency and characteristics of coagulopathy in trauma patients treated with a low- or high-plasma-content massive transfusion protocol. Am J Clin Pathol. 2011;136(3):364–70.
Brown LM, Aro SO, Cohen MJ. A high fresh frozen plasma: packed red blood cell transfusion ratio decreases mortality in all massively transfused trauma patients regardless of admission international normalized ratio. J Trauma Acute Care Surg. 2011;71(2):S358–63.
Straat M, Müller MCA, Meijers JCM, Arbous MS, Spoelstra de Man AME, Beurskens CJP, et al. Effect of transfusion of fresh frozen plasma on parameters of endothelial condition and inflammatory status in non-bleeding critically ill patients: a prospective substudy of a randomized trial. Crit Care. 2015;19(1):163.
Esmon CT. The protein C anticoagulant pathway. Arterioscler Thromb. 1992;12(2):135–45.
Sturn DH, Kaneider NC, Feistritzer C, Djanani A, Fukudome K, Wiedermann CJ. Expression and function of the endothelial protein C receptor in human neutrophils. Blood. 2003;102(4):1499–505.
Joyce DE, Gelbert L, Ciaccia A, DeHoff B, Grinnell BW. Gene expression profile of antithrombotic protein c defines new mechanisms modulating inflammation and apoptosis. J Biol Chem. 2001;276(14):11199–203.
Feistritzer C, Riewald M. Endothelial barrier protection by activated protein C through PAR1-dependent sphingosine 1-phosphate receptor-1 crossactivation. Blood. 2005;105(8):3178–84.
Murakami K, Okajima K, Uchiba M, Johno M, Nakagaki T, Okabe H, et al. Activated protein C attenuates endotoxin-induced pulmonary vascular injury by inhibiting activated leukocytes in rats. Blood. 1996;87(2):642–7.
Robriquet L, Collet F, Tournoys A, Prangère T, Nevière R, Fourrier F, et al. Intravenous administration of activated protein C in pseudomonas-induced lung injury: impact on lung fluid balance and the inflammatory response. Respir Res. 2006;22:7.
Bernard GR, Vincent JL, Laterre PF, LaRosa SP, Dhainaut JF, Lopez-Rodriguez A, et al. Efficacy and safety of recombinant human activated protein C for severe sepsis. N Engl J Med. 2001;344(10):699–709.
Abraham E, Laterre PF, Garg R, Levy H, Talwar D, Trzaskoma BL, et al. Drotrecogin alfa (Activated) for adults with severe sepsis and a low risk of death. N Engl J Med. 2005;353(13):1332–41.
Nadel S, Goldstein B, Williams MD, Dalton H, Peters M, Macias WL, Abd-Allah SA, Levy H, Angle R, Wang D, Sundin DP. Drotrecogin alfa (activated) in children with severe sepsis: a multicentre phase III randomised controlled trial. The Lancet. 2007;369(9564):836–43.
Sinha P, He J, Matthay MA, Churpek MM, Ware LB, Calfee CS. D16 advancing the science of ards and acute respiratory failure / Mini symposium the hyperinflammatory and hypoinflammatory phenotypes have divergent clinical outcomes in sepsis and differential response to activated protein C. [cited 2023 Jul 16]; Available from: www.atsjournals.org.
Funding
Medical Research Council. British Journal of Anaesthesia-Royal College of Anaesthetists.
Author information
Authors and Affiliations
Contributions
Conception and design: RR, DM, CO, JS. Drafting of manuscript: RR and JS. Critical revision of manuscript: RR, DM, CO, JS.
Corresponding author
Ethics declarations
Ethical approval and consent to participate
Not Applicable.
Competing interests
RR reports no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
PubMed was searched for articles published from database inception to 1st September 2022, by use of the terms “sepsis”, “endothelial dysfunction”, “capillary leak”. Relevant references cited in papers identified also reviewed. The therapeutic agents selected for discussion were those for which the greatest body of pre-clinical and clinical evidence was identified.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
McMullan, R.R., McAuley, D.F., O’Kane, C.M. et al. Vascular leak in sepsis: physiological basis and potential therapeutic advances. Crit Care 28, 97 (2024). https://doi.org/10.1186/s13054-024-04875-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13054-024-04875-6