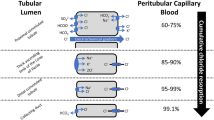
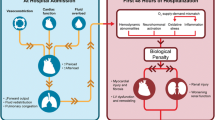
Abstract
Hyponatremia is a common electrolyte abnormality with important prognostic and therapeutic implications. It might exert detrimental effects on various organ systems including the central nervous system (CNS), bone, and heart along with its potential association with poor quality of life. These adverse effects might be largely mediated through a variety of mechanisms including osmotic stress, dysfunctional transmembrane exchangers, and enhanced oxidative stress.
Interestingly, hyponatremia might also have an important association with takotsubo syndrome (TTS) that has been universally considered as a reversible form of cardiomyopathy usually emerging in response to various stressors. In this context, severe hyponatremia was previously reported to serve as a direct trigger of TTS evolution largely through its potential impact on CNS and heart. However, pathogenetic and clinical implications of hyponatremia still need to be thoroughly evaluated in patients with TTS. This paper aims to analyze the clinical features of published cases with TTS primarily triggered by hyponatremia and also aims to discuss the association between hyponatremia and TTS from a broader perspective.



Similar content being viewed by others
Availability of data and materials
Not applicable.
References
Yalta K, Yilmaztepe M, Zorkun C (2018) Left Ventricular dysfunction in the setting of takotsubo cardiomyopathy: a review of clinical patterns and practical implications. Card Fail Rev 4(1):14–20
Santillo E, D'Onofrio P, Marini L et al (2022) Takotsubo syndrome presenting with left bundle branch block in an octogenarian. Is hyponatremia a pathogenic "common ground"? J Geriatr Cardiol 19(8):622–625
Perera I, Rajapakse S, De Silva ST (2020) Severe hyponatremia-induced stress cardiomyopathy: a case report and review of literature. Case Rep Cardiol 31(2020):2961856. https://doi.org/10.1155/2020/2961856.PMID:32292605;PMCID:PMC7150708
Yalta K, Yetkin E, Yalta T (2021) Systemic inflammation in patients with takotsubo syndrome: a review of mechanistic and clinical implications. Monaldi Arch Chest Dis 91(2). https://doi.org/10.4081/monaldi.2021.1718. PMID: 33728882
Lyon AR, Citro R, Schneider B et al (2021) Pathophysiology of takotsubo syndrome: JACC state-of-the-art review. J Am Coll Cardiol 77(7):902–921
Dodoo SN, Agyemang-Sarpong A, Taka N et al (2022) Takotsubo cardiomyopathy in the setting of severe hyponatremia and beer potomania: a case report. Clin Case Rep 10(12):e6717. https://doi.org/10.1002/ccr3.6717
Murguía-Aranda A, Castañón-González JA, Shuchleib-Cukiert M et al (2021) Takotsubo (stress cardiomyopathy) syndrome and inappropriate antidiuretic hormone secretion. Cir Cir 89(3):394–398
Nakamura M, Nagamine T (2019) Severe lamotrigine-induced hyponatremia associated with takotsubo cardiomyopathy. Innov Clin Neurosci 16(7–08):32–34
Yasutomi M, Nakamura S, Makino Y et al (2020) A Case of Takotsubo cardiomyopathy with a rare transition pattern of left ventricular wall motion abnormality. Am J Case Rep 21:e926670. https://doi.org/10.12659/AJCR.926670
Simsek EC, Emren SV, Ozdogan O (2018) Unusual combined cause of takotsubo cardiomyopathy: Hyponatremia and seizure. North Clin Istanb 6(3):304–307
Jha KK, Kumar M, Jha U, Desar S (2016) Takotsubo cardiomyopathy in a patient with SIADH. Int J Cardiol 225:342–344
Cecconi A, Franco E, de Agustín JA et al (2016) Hyponatremia-induced stress cardiomyopathy due to psychogenic polydipsia. Int J Cardiol 202:618–620
Patnaik S, Punjabi C, Nathan R et al (2015) Bland and broken hearted: a case of hyponatremia induced Tako-tsubo cardiomyopathy. Int J Cardiol 187:267–271
Chikata A, Omi W, Saeki T et al (2014) Repeated pacemaker dysfunction in a patient with recurrent takotsubo cardiomyopathy precipitated by hyponatremia. Int J Cardiol 170(3):443–444
Sagiv O, Vukelic S, Czak S et al (2012) Apical ballooning syndrome associated with isolated severe hyponatremia: case report and suggested pathophysiology. Rev Cardiovasc Med 13(4):e198-202
Santos M, Dias V, Meireles A et al (2011) Hyponatremia–an unusual trigger of takotsubo cardiomyopathy. Rev Port Cardiol 30(11):845–848
Kawano H, Matsumoto Y, Arakawa S et al (2011) Takotsubo cardiomyopathy in a patient with severe hyponatremia associated with syndrome of inappropriate antidiuretic hormone. Intern Med 50(7):727–732
AbouEzzeddine O, Prasad A (2010) Apical ballooning syndrome precipitated by hyponatremia. Int J Cardiol 145(1):e26–e29
Andreozzi F, Cuminetti G, Karmali R, Kamgang P (2018) Electrolyte disorders as triggers for takotsubo cardiomyopathy. Eur J Case Rep Intern Med 5(4):000760
Lemke DM, Hussain SI, Wolfe TJ et al (2008) Takotsubo cardiomyopathy associated with seizures. Neurocrit Care 9(1):112–117
Worthley MI, Anderson TJ (2007) Transient left ventricular apical ballooning syndrome following a hyponatraemic seizure. Int J Cardiol 115(3):e102–e104
Vriz O, Citro R, Martina S et al (2009) Tako-tsubo syndrome and hypovolemia. Monaldi Arch Chest Dis 72(3)
Purdy A, Ren B (2018) A broken heart: a rare complication of hyponatremia. J Med Cases 9(5):147–150
Vereeke J, Lefebvre C, Laterre PF et al (2021) Takotsubo syndrome associated with isolated hyponatremia: case report. In: J Integr Cardiol Open Access 4(1):1–3. https://doi.org/10.31487/j.jicoa.2021.01.07
Kim JT, Seo SH, Lee SH et al (2015) Stress-induced cardiomyopathy associated with non-small cell lung cancer presenting as hyponatremia. Ewha Med J 38(2):90–93
Yenigella S, Kaur N (2018) An usual trigger for stress cardiomyopathy. J Med Cases 9(12):386–389
Hoorn EJ, Zietse R (2017) Diagnosis and Treatment of Hyponatremia: Compilation of the Guidelines. J Am Soc Nephrol 28(5):1340–1349
Schrier RW, Sharma S, Shchekochikhin D (2013) Hyponatraemia: more than just a marker of disease severity? Nat Rev Nephrol 9(1):37–50
Batta A, Gupta AK, Singal G et al (2022) Autoimmune polyendocrine syndrome II presenting paradoxically as Takotsubo cardiomyopathy: a case report and reappraisal of pathophysiology. Egypt Heart J 74(1):82
Bagnall T, Tow YR, Bunce N, Astroulakis Z (2021) Takotsubo cardiomyopathy associated with adrenal insufficiency in the context of long-term steroid use mimicking acute coronary syndrome. BMJ Case Rep 14(1):e234983
Vieira A, Batista B, de Abreu TT (2018) Iatrogenic takotsubo cardiomyopathy secondary to norepinephrine by continuous infusion for shock. Eur J Case Rep Intern Med 5(7):000894
Singh G, Manickam A, Sethuraman M, Rathod RC (2015) Takotsubo cardiomyopathy in a patient with pituitary adenoma and secondary adrenal insufficiency. Indian J Crit Care Med 19(12):731–734
Murakami M, Matsushita N, Arai R et al (2012) Isolated adrenocorticotropin deficiency associated with delirium and takotsubo cardiomyopathy. Case Rep Endocrinol 2012:580481
Yalta K, Yalta T, Sivri N et al (2013) Copeptin and cardiovascular disease: a review of a novel neurohormone. Int J Cardiol 167(5):1750–1759
Gupta S, Goyal P, Idrees S et al (2018) Association of endocrine conditions with takotsubo cardiomyopathy: a comprehensive review. J Am Heart Assoc 7(19):e009003
Aikins AO, Little JT, Rybalchenko N, Cunningham JT (2022) Norepinephrine innervation of the supraoptic nucleus contributes to increased copeptin and dilutional hyponatremia in male rats. Am J Physiol Regul Integr Comp Physiol 323(5):R797–R809
Yalta K (2012) Serum copeptin/NT-proBNP ratio: a more reliable index of absolute endogenous stress and prognosis during the course of Tako-tsubo cardiomyopathy? Int J Cardiol 154(3):376–377
Wilson MF, Brackett DJ, Archer LT, Hinshaw LB (1980) Mechanisms of impaired cardiac function by vasopressin. Ann Surg 191(4):494–500
Boyle WA 3rd, Segel LD (1986) Direct cardiac effects of vasopressin and their reversal by a vascular antagonist. Am J Physiol 251(4 Pt 2):H734–H741
Indrambarya T, Boyd JH, Wang Y et al (2009) Low-dose vasopressin infusion results in increased mortality and cardiac dysfunction following ischemia-reperfusion injury in mice. Crit Care 13:R98. https://doi.org/10.1186/cc7930
De Picker L, Van Den Eede F, Dumont G et al (2014) Antidepressants and the risk of hyponatremia: a class-by-class review of literature. Psychosomatics 55(6):536–547
Whiskey E, Taylor D (2013) A review of the adverse effects and safety of noradrenergic antidepressants. J Psychopharmacol 27(8):732–739
Karim MR, Jawairia M, Rahman S et al (2011) Cocaine-associated acute severe hyponatremia. Clin Nephrol 75(Suppl 1):11–15 (PMID: 21269586)
Patel A, Mirza N, Ali R et al (2022) Takotsubo cardiomyopathy after cocaine intoxication. Eur J Case Rep Intern Med 9(9):003457
Neil CJ, Chong CR, Nguyen TH, Horowitz JD (2012) Occurrence of Tako-Tsubo cardiomyopathy in association with ingestion of serotonin/noradrenaline reuptake inhibitors. Heart Lung Circ 21(4):203–205
Atzori M, Cuevas-Olguin R, Esquivel-Rendon E et al (2016) Locus ceruleus norepinephrine release: a central regulator of CNS spatio-temporal activation? Front Synaptic Neurosci 8:25
Rodovalho GV, Franci CR, Morris M, Anselmo-Franci JA (2006) Locus coeruleus lesions decrease oxytocin and vasopressin release induced by hemorrhage. Neurochem Res 31(2):259–266
Kounis NG, Mplani V, de Gregorio C, Koniari I (2023) Attack the ATAK; a challenging contemporary complex: pathophysiologic, therapeutic, and preventive considerations. Balkan Med J. https://doi.org/10.4274/balkanmedj.galenos.2023.2023-4-96. Epub ahead of print. PMID: 37218727
Nahar N, Akhter N (2009) Effect of carvedilol on adrenaline-induced changes in serum electrolytes in rat. Bangladesh Med Res Counc Bull 35(3):105–109
Myburgh JA, Upton RN, Grant C, Martinez A (2002) The cerebrovascular effects of adrenaline, noradrenaline and dopamine infusions under propofol and isoflurane anaesthesia in sheep. Anaesth Intensive Care 30(6):725–733
Giuliani C, Peri A (2014) Effects of hyponatremia on the brain. J Clin Med 3(4):1163–1177
Adrogué HJ, Madias NE (2000) Hyponatremia. N Engl J Med 342(21):1581–1589
Okada Y, Sato K, Numata T (2009) Pathophysiology and puzzles of the volume-sensitive outwardly rectifying anion channel. J Physiol 587(Pt 10):2141–2149
Shigeri Y, Seal RP, Shimamoto K (2004) Molecular pharmacology of glutamate transporters. EAATs and VGLUTs Brain Res Rev 45(3):250–265
Tahsili-Fahadan P, Geocadin RG (2017) Heart-brain axis: effects of neurologic injury on cardiovascular function. Circ Res 120(3):559–572
Yalta K, Ozkan U, Yalta T, Yetkin E (2021) Cardiac myxoma as a potential trigger of takotsubo cardiomyopathy: a brief review on mechanistic and clinical perspectives. Monaldi Arch Chest Dis 92(1)
Yalta K, Taylan G, Yalta T, Yetkin E (2020) Takotsubo cardiomyopathy in the setting of multiple sclerosis: a multifaceted phenomenon with important implications. Monaldi Arch Chest Dis 90(3)
Hiestand T, Hänggi J, Klein C et al (2018) Takotsubo syndrome associated with structural brain alterations of the limbic system. J Am Coll Cardiol 71(7):809–811
Fujisawa H, Sugimura Y, Takagi H et al (2016) Chronic hyponatremia causes neurologic and psychologic impairments. J Am Soc Nephrol 27(3):766–780
Yalta K, Yetkin E, Yalta T (2021) Takotsubo cardiomyopathy: an obscure cause of emerging cardiovascular manifestations in the setting of Bickerstaff’s brainstem encephalitis. Neurol Sci 42(3):1181–1183
Kolar F, Cole WC, Ostadal B, Dhalla NS (1990) Transient inotropic effects of low extracellular sodium in perfused rat heart. Am J Physiol 259(3 Pt 2):H712–H719
Mouallem M, Friedman E, Shemesh Y et al (1991) Cardiac conduction defects associated with hyponatremia. Clin Cardiol 14(2):165–168
Barsony J, Sugimura Y, Verbalis JG (2011) Osteoclast response to low extracellular sodium and the mechanism of hyponatremia-induced bone loss. J Biol Chem 286(12):10864–10875
Fibbi B, Marroncini G, Anceschi C et al (2021) Hyponatremia and oxidative stress. Antioxidants (Basel) 10(11):1768. https://doi.org/10.3390/antiox10111768
Barsony J, Manigrasso MB, Xu Q et al (2013) Chronic hyponatremia exacerbates multiple manifestations of senescence in male rats. Age (Dordr) 35(2):271–288
Oniki T, Teshima Y, Nishio S et al (2019) Hyponatremia aggravates cardiac susceptibility to ischaemia/reperfusion injury. Int J Exp Pathol 100(5–6):350–358
Manousek J, Kala P, Lokaj P et al (2021) Oxidative stress in takotsubo syndrome-is it essential for an acute attack? Indirect evidences support multisite impact including the calcium overload-energy failure hypothesis. Front Cardiovasc Med 8:732708
Yalta K, Ozturk C, Gok M, Yalta T (2023) Takotsubo syndrome during breastfeeding: Further insights into prolactin and its implications. Rev Port Cardiol S0870–2551(23)00188–9. English, Portuguese. https://doi.org/10.1016/j.repc.2022.11.005. Epub ahead of print. PMID: 37019281
Ciutac AM, Dawson D (2021) The role of inflammation in stress cardiomyopathy. Trends Cardiovasc Med 31(4):225–230
Lugrin J, Rosenblatt-Velin N, Parapanov R, Liaudet L (2014) The role of oxidative stress during inflammatory processes. Biol Chem 395(2):203–230
Yalta K, Yilmaz MB, Yalta T et al (2020) Late versus early myocardial remodeling after acute myocardial infarction: a comparative review on mechanistic insights and clinical implications. J Cardiovasc Pharmacol Ther 25(1):15–26
Pongratz G, Straub RH (2014) The sympathetic nervous response in inflammation. Arthritis Res Ther 16(6):504
Dhalla NS, Adameova A, Kaur M (2010) Role of catecholamine oxidation in sudden cardiac death. Fundam Clin Pharmacol 24(5):539–546
Dhalla NS (2018) Formation of aminochrome leads to cardiac dysfunction and sudden cardiac death. Circ Res 123(4):409–411
Yates JC, Dhalla NS (1975) Induction of necrosis and failure in the isolated perfused rat heart with oxidized isoproterenol. J Mol Cell Cardiol 7(11):807–816
Sethi R, Adameova A, Dhalla KS et al (2009) Modification of epinephrine-induced arrhythmias by N-acetyl-L-cysteine and vitamin E. J Cardiovasc Pharmacol Ther 14(2):134–142
Wang J, Zhou W, Yin X (2019) Improvement of hyponatremia is associated with lower mortality risk in patients with acute decompensated heart failure: a meta-analysis of cohort studies. Heart Fail Rev 24(2):209–217
Leier CV, Dei Cas L, Metra M (1994) Clinical relevance and management of the major electrolyte abnormalities in congestive heart failure: hyponatremia, hypokalemia, and hypomagnesemia. Am Heart J 128(3):564–574
Acknowledgements
Figures 2 and 3 were created with BioRender.com.
Author information
Authors and Affiliations
Contributions
K.Y.: substantial writing, concept, design, literature search, preparation of table and figures; O.P.: writing, concept, design, literature search, preparation of table and figures; M.G.: writing, concept, design, literature search, preparation of table and figures; E.Y.: writing, concept, design, literature search, preparation of table and figures. All authors approved the final version of the manuscript.
Corresponding author
Ethics declarations
Ethical approval
Not applicable.
Consent to participate
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Yalta, K., Palabıyık, O., Gurdogan, M. et al. Hyponatremia and takotsubo syndrome: a review of pathogenetic and clinical implications. Heart Fail Rev 29, 27–44 (2024). https://doi.org/10.1007/s10741-023-10344-z
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10741-023-10344-z