Abstract
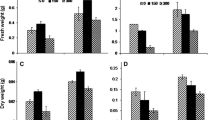
Greenhouse pot experiments were conducted to investigate genotypic differences in response to drought stress between Tibetan wild barley genotypes (XZ5 and XZ150, drought-tolerant; XZ54 and XZ147, drought-sensitive) and cv ZAU3. Drought stress of 4 % soil moisture content (SMC) significantly decreased water potential (WP) and osmotic potential (OP), while increased water saturation deficit (WSD) and the bound water content (BWC) in leaves, with the least decrease/increase in XZ5, which recorded the highest levels in WP and OP but the lowest in WSD and BWC under 4 % SMC. Under 15 % SMC, when compared with control, XZ5 and XZ150 had more elevation in soluble sugar content relative to the other 3 genotypes, while XZ5 had the least increase in soluble protein. Under 4 % SMC, endogenous ABA content increased more in XZ5 and XZ150 than in the other three genotypes, but proline content increased least in XZ5. After rewatering, the transpiration rate increased in sensitive genotypes but decreased in tolerant genotypes. The less elevated MDA accumulation was observed in XZ5 and XZ150 with higher POD and CAT activities under 15 and 4 % SMC than in the other three genotypes. Drought stress of 4 % SMC significantly up-regulated the expression levels of CAT1 and Cu/ZnSOD in XZ5 and MnSOD in XZ150.Our results indicated that drought tolerance of wild barley XZ5 is mainly associated with the osmo-regulation of soluble sugar and stomatal regulation of ABA. This mechanism could be applied to improve the drought tolerance of cultivated barley and the further marker-assisted breeding.




Similar content being viewed by others
References
Apel KH, Hirt H (2004) Reactive oxygen species: metabolism, oxidative stress, and signal transduction. Annu Rev Plant Biol 55:373–399
Ashraf M, Foolad MR (2007) Roles of glycine betaine and proline in improving plant abiotic stress resistance. Environ Exp Bot 59(2):206–216
Ashraf M, Ahmad A, McNeilly T (2001) Growth and photosynthetic characteristics in pearl millet under water stress and different potassium supply. Photosynthetica 39(3):389–394
Bornare SS, Prasad LC, Prasad R, Lal JP (2012) Perspective of barley drought tolerance; methods and mechanisms comparable to other cereals. J Progress Agric 3:68–70
Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72:248–254
Ceccarelli S (2010) Drought and drought resistance. Encycl Biotechnol Agric Food 1:205–207
Centritto M (2005) Photosynthetic limitations and carbon partitioning in cherry in response to water deficit and elevated [CO2]. Agric Ecosyst Environ 106(2–3):233–242
Cochard H, Coll L, Le Roux X, Ameglio T (2002) Unraveling the effects of plant hydraulics on stomatal closure during water stress in walnut. Plant Physiol 128(1):282–290
Colom MR, Vazzana C (2003) Photosynthesis and PSII functionality of drought-resistant and drought-sensitive weeping lovegrass plants. Environ Exp Bot 49(2):135–144
Cornic G, Fresneau C (2002) Photosynthetic carbon reduction and carbon oxidation cycles are the main electron sinks for photosystem II activity during a mild drought. Ann Bot 89:887–894
Crozier A, Sandberg G, Monteiro AM, Sundberg B (1986) The use of immunological techniques in plant hormone analysis. In: Bopp M (ed) Plant growth substances 1985. Springer, Berlin, pp 13–21
de Souza TC, Magalhães PC, de Castro EM, Carneiro NP, Padilha FA, Júnior CCG (2014) ABA application to maize hybrids contrasting for drought tolerance: changes in water parameters and in antioxidant enzyme activity. Plant Growth Regul 73(3):205–217
Desikan R, Cheung MK, Bright J, Henson D, Hancock JT, Neill SJ (2004) ABA, hydrogen peroxide and nitric oxide signaling in stomatal guard cells. J Exp Bot 55:205–212
Ellis RP, Forster BP, Robinson D, Handley LL, Gordon DC, Russell JR, Powell W (2000) Wild barley: a source of genes for crop improvement in the 21st century? J Exp Bot 342(51):9–17
Flexas J, Bota J, Loreto F, Cornic G, Sharkey TD (2004) Diffusive and metabolic limitations to photosynthesis under drought and sanlinity in C3 plants. Plant Biol 6:269–279
Foyer CH, Noctor G (2005) Oxidant and antioxidant signalling in plants: a re-evaluation of the concept of oxidative stress in a physiological context. Plant, Cell Environ 28:1056–1071
Gupta N, Thind SK, Bains NS (2014) Glycine betaine application modifies biochemical attributes of osmotic adjustment in drought stressed wheat. Plant Growth Regul 72(3):221–228
Hartung W, Sauter A, Hose E (2002) Abscisic acid in the xylem: where does it come from, where does it go to? J Exp Bot 53:27–37
Hong ZL, Lakkineni K, Zhang ZM, Verma DPS (2000) Removal of feedback inhibition of Δ1-pyrroline-5-carboxylate synthetase results in increased proline accumulation and protection of plants from osmotic stress. Plant Physiol 122:1129–1136
Kaur K, Kaur N, Gupta AK, Singh I (2013) Exploration of the antioxidative defense system to characterize chickpea genotypes showing differential response towards water deficit conditions. Plant Growth Regul 70(1):49–60
Kautz B, Noga G, Hunsche M (2014) Sensing drought-and salinity-imposed stresses on tomato leaves by means of fluorescence techniques. Plant Growth Regul 73(3):279–288
Krause GH, Wei E (1991) Chlorophyll fluorescence and photosynthesis: the basics. Ann Rev Plant Physiol Plant Mol Biol 42:313–349
Liang M, Chen D, Lin M, Zheng Q, Huang Z, Lin Z, Zhao G (2014) Isolation and characterization of two DREB1 genes encoding dehydration-responsive element binding proteins in chicory (Cichorium intybus). Plant Growth Regul 73(1):45–55
Nayyar H, Walia DP (2003) Water stress induced proline accumulation in contrasting wheat genotypes as affected by calcium and abscisic acid. Biol Plant 46:275–279
Pennisi E (2008) The blue revolution, drop by drop, gene by gene. Science 320:171–173
Pinheiro HA, DaMatta FM, Chaves ARM, Fontes EPB, Loureiro ME (2004) Drought tolerance in relation to protection against oxidative stress in clones of Coffea canephora subjected to long-term drought. Plant Sci 167(6):1307–1314
Ranney TG, Bassuk NL, Whitlow TH (1991) Osmotic adjustment and solute constituents in leaves and roots of water-stressed cherry (Prunus) trees. J Am Soc Hortic Sci 116(4):684–688
Saradadevi R, Bramley H, Siddique KHM, Edwards E, Palta JA (2014) Contrasting stomatal regulation and leaf ABA concentrations in wheat genotypes when split root systems were exposed to terminal drought. Field Crop Res 162:77–86
Singh T, Paleg I, Aspinall D (1973) Stress metabolism I. Nitrogen metabolism and growth in the barley plant during water stress. Aust J Biol Sci 26:45–56
Song XS, Hu WH, Mao WH, Ogweno JO, Zhou YH, Yu JQ (2005) Response of ascorbate peroxidase isoenzymes and ascorbate regeneration system to abiotic stresses in Cucumis sativus L. Plant Physiol Biochem 43(12):1082–1088
Szalai G, Kellős T, Galiba G, Kocsy G (2009) Glutathione as an antioxidant and regulatory molecule in plants under abiotic stress conditions. J Plant Growth Regul 28(1):66–80
Tang QY, Feng MG (2002) DPS data processing system for practical statistics. Science Press, Bejing
Wang W, Vinocur B, Shoseyov O, Altman A (2004) Role of plant heat-shock proteins and molecular chaperones in the abiotic stress response. Trends Plant Sci 9(5):244–252
Wang A, Yu Z, Ding Y (2009) Genetic diversity analysis of wild close relatives of barley from Tibet and the Middle East by ISSR and SSR markers. C R Biol 332(4):393–403
Wu FB, Zhang GP, Dominy P (2003) Four barley genotypes respond differently to cadmium: lipid peroxidation and activities of antioxidant capacity. Environ Exp Bot 50(1):67–78
Yemm EW, Willis AJ (1954) The estimation of carbohydrates in plant extracts by anthrone. Biochem J 57:508–514
Zhang XZ (1992) The measurement and mechanism of lipid peroxidation and SOD, POD and CAT activities in biological system. In: Zhang XZ (ed) Research methodology of crop physiology. Agriculture Press, Beijing, pp 208–211
Zhang X, Zhang L, Dong FC, Gao JF, Galbraith DW, Song CP (2001) Hydrogen peroxide is involved in abscisic acid-induced stomatal closure in Vicia faba. Plant Physiol 126:1438–1448
Zhao J, Sun HY, Dai HX, Zhang GP, Wu FB (2010) Difference in response to drought stress among Tibet wild barley genotypes. Euphytica 172(3):395–403
Author information
Authors and Affiliations
Corresponding author
Additional information
Mian Zhang and Zhu-Qun Jin have contributed equally to this article.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Zhang, M., Jin, ZQ., Zhao, J. et al. Physiological and biochemical responses to drought stress in cultivated and Tibetan wild barley. Plant Growth Regul 75, 567–574 (2015). https://doi.org/10.1007/s10725-014-0022-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10725-014-0022-x