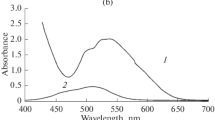
The solid-phase synthesis of multicomponent bismuth tantalates and niobates with pyrochloric structure (space group Fd-3m), containing equimolar amounts of transition 3d-element atoms, is demonstrated. The composition of such pyrochlores can be described by the stoichiometric formula Bi2Cr1/6Mn1/6Fe1/6Co1/6Ni1/6Cu1/6Ta(Nb)2O9±∆. An aspect of the synthesis of pyrochlores is a multi-stage high-temperature process of heat treatment of samples in the range of 650 – 1050°C for 60 h. For pyrochlore based on bismuth tantalate, an impurity phase of triclinic bismuth orthotantalate β-BiTaO4 (sp. gr. P-1) is formed in trace quantities. Complex pyrochlore based on bismuth niobate is characterized by a dense, low-porous microstructure, in contrast to open, porous tantalum pyrochlore with average grain size equal to about 2 µm. The unit cell parameter for niobium- and tantalum-containing pyrochlores is equal to 10.4927 (10.4922) Å respectively.




Similar content being viewed by others
References
S. Murugesan, M. N. Huda, Y. Yen, et al., “Band-engineered bismuth titanate pyrochlores for visible light photocatalysis,” J. Phys. Chem. C, 114(23), 10598 – 10605 (2010).
J. Pandey, V. Shrivastava, and R. Nagarajan, “Metastable Bi2Zr2O7 with pyrochlore-like structure: Stabilization, oxygen ion conductivity, and catalytic properties,” Inorg. Chem., 57(21), 13667 – 13678 (2018).
C. C. Khaw, K. B. Tan, and C. K. Lee, “High temperature dielectric properties of cubic bismuth zinc tantalate,” Ceram. Int., 35(4), 1473 – 1480 (2009).
J. E. Greedan, “Frustrated rare earth magnetism: Spin glasses, spin liquids and spin ices in pyrochlore oxides,” J. Alloys Comp., 408 – 412, 444 – 455 (2006).
N. A. Zhuk, M. G. Krzhizhanovskaya, A. V. Koroleva, et al., “Thermal expansion, XPS spectra, and structural and electrical properties of a new Bi2NiTa2O9 pyrochlore,” Inorg. Chem., 60(7), 4924 – 4934 (2021).
T. A. Vanderah, M. W. Lufaso, A. U. Adler, et al., “Subsolidus phase equilibria and properties in the system Bi2O3:Mn2O3±xNb2O5,” J. Solid State Chem., 179(11), 3467 – 3477 (2006).
T. A. Vanderah, T. Siegrist, M. W. Lufaso, et al., “Phase formation and properties in the system Bi2O3:2CoO1+xNb2O5,” Eur. J. Inorg. Chem., 2006(23), 4908 – 4914 (2006).
M. P. Chon, K. B. Tan, C. C. Khaw, et al., “Subsolidus phase equilibria and electrical properties of pyrochlores in the Bi2O3–CuO–Ta2O5 ternary system,” J. Alloys Comp., 675, 116 – 127 (2016).
M. Valant and D. Suvorov, “The Bi2O3–Nb2O5–NiO phase diagram,” J. Am. Ceram. Soc., 88(9), 2540 – 2543 (2005).
M.W. Lufaso, T. A. Vanderah, I. M. Pazos, et al., “Phase formation, crystal chemistry, and properties in the system Bi2O3–Fe2O3–Nb2O5,” J. Sol. St. Chem., 179(12), 3900 – 3910 (2006).
F. A. Jusoh, K. B. Tan, Z. Zainal, et al., “Novel pyrochlores in the Bi2O3–Fe2O3–Ta2O5 (BFT) ternary system: synthesis, structural and electrical properties,” J. Mater. Res. Techn., 9, 11022 – 11034 (2020).
P. Y. Tan, K. B. Tan, C. Khaw, et al., “Structural and electrical properties of bismuth magnesium tantalate pyrochlores,” Ceram. Int., 38, 5401 – 5409 (2012).
N. A. Zhuk, M. G. Krzhizhanovskaya, A. V. Koroleva, et al., “Cu, Mg co-doped bismuth tantalate pyrochlores: crystal structure, XPS spectra, thermal expansion, and electrical properties,” Inorg. Chem., 61, 4270 – 4282 (2022).
M. A. Subramanian, G. Aravamudan, and G. V. Subba Rao, “Oxide pyrochlores – a review,” Prog. Solid State Chem., 15(2), 55 – 143 (1983).
R. A. McCauley, “Structural characteristics of pyrochlore formation,” J. Appl. Phys., 51(1), 290 – 294 (1980).
L. G. Akselrud, Yu. N. Grin, P. Yu. Zavalij, et al., “CSD-universal program package for single crystal or powder structure data treatment,” in: Thes. Rep. XII EUR. Crystallogr. Meet., Moscow (1989), Vol. 3, p. 155.
N. A. Zhuk and M. G. Krzhizhanovskaya, “Thermal expansion of bismuth magnesium tantalate and niobate pyrochlores,” Ceram. Int., 47(21), 30099 – 30105 (2021).
V. A. Muraviev, B. A. Makeev, M. G. Krzhizhanovskaya, et al. “Synthesis of Bi2NiTa2O9 with a pyrochlore structure,” Glass Ceram., 79(1 – 2), 70 – 74 (2022).
N. A. Zhuk, M. G. Krzhizhanovskaya, V. A. Belyy, et al., “Phase transformations and thermal expansion of α- and β-BiTaO4 and the high-temperature modification of γ-BiTaO4,” Chem. Mater., 32(13), 5493 – 5501 (2020).
N. A. Zhuk, M. G. Krzhizhanovskaya, A. V. Koroleva, et al., “Spectroscopic characterization of cobalt doped bismuth tantalate pyrochlore,” Solid State Sci., 125, 106820 (2022).
Author information
Authors and Affiliations
Corresponding author
Additional information
Translated from Steklo i Keramika, No. 10, pp. 34 – 39, October, 2022.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Parshukova, K.N., Rylchenko, E.P., Muravyov, V.A. et al. Synthesis of Multicomponent Compounds with Pyrochloric Structure. Glass Ceram 79, 418–421 (2023). https://doi.org/10.1007/s10717-023-00523-7
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10717-023-00523-7