Abstract
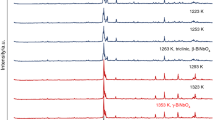
High-temperature transformation of β-PtBi2, a synthetic analogue of insizwaite, was studied under oxidizing conditions (on air). The behavior of the sample upon heating up to 660°C and cooling was studied by differential thermal analysis (DTA) and high-temperature X-ray powder diffraction. It was shown that polymorphic transitions of β-PtBi2 into high-temperature γ- and δ-polymorphs do not occur under thermal treatment in the oxidizing conditions (on air). The DTA curve shows two exothermic peaks at 318 and 620°C. The total weight change in the temperature range studied is +10.5%. No thermal reactions or weight changes were recorded upon cooling of the sample. According to the high-temperature diffraction experiment in situ, the decomposition of the synthesized phase into Pt and Bi was recorded with their subsequent oxidation to bismuth oxide Bi2O3 with the bismite-type structure and double platinum and bismuth oxide with the pyrochlore-type structure Bi2Pt2O7. The study by electron microprobe analysis showed that the grains of the transformed insizwaite are a finely dispersed mixture of two oxides.








Similar content being viewed by others
REFERENCES
H. Okamoto, J. Phase Equilib. 12 (2), 207–210 (1991).
Y. C. Bhatt and K. Schubert, Z. Metallkd. 71, 581–583 (1980).
S. Furuseth, K. Selte, and A. Kjekshus, Acta Chem. Scand. 19, 735–741 (1965).
N. E. Brese and H. G. von Schnering, Z. Anorg. Allg. Chem. 620, 393–404 (1994).
G. Shipunov, I. Kovalchuk, B. R. Piening, V. Labracherie, A. Veyrat, D. Wolf, A. Lubk, S. Subakti, R. Giraud, J. Dufouleur, S. Shokri, F. Caglieris, C. Hess, D. V. Efremov, B. Buchner, and S. Aswartham, Phys. Rev. Mater. 4 (12), 124202–124210 (2020).
Y. C. Bhatt and K. Schubert, Z. Metallkd. 71, 550–553 (1980).
L. J. Cabri and D. C. Harris, Mineral. Mag. 38, 794–800 (1972).
L. J. Cabri and J. H. G. Laflamme, Econ. Geol. 71 (7), 1159–1195 (1976).
C. Li and A. J. Naldrett, Can. Mineral. 31 (1), 31–44 (1993).
T. Augé, É. Gloaguen, M. Chevillard, and L. Bailly, Mineral. Mag. 82 (3), 593–624 (2018).
D. A. Holwell and I. McDonald, Contrib. Mineral. Petrol. 154 (2), 171–190 (2007).
E. M. Spiridonov, E. A. Kulagov, A. A. Serova, I. M. Kulikova, N. N. Korotaeva, E. V. Sereda, I. N. Tushentsova, S. N.Belyakov, and N. N. Zhukov, Geol. Ore Deposits 57 (5), 402 (2015).
N. N. Zhuravlev and A. A. Stepsanov, Kristallografiya 7, 310–311 (1962).
C. Frondel, Am. Mineral. 28 (9–10), 521–535 (1943).
G. Malmros, Acta Chem. Scand. 24 (2), 384–396 (1970).
A. W. Sleight, Mater. Res. Bull. 9 (9), 1177–1184 (1974).
E. G. Eremenko and S. V. Petrov, in All-Russ. Conf. with International Participation “Problems of Geology and Exploitation of Platinum Metal Deposits” (The Ist Readings devoted to Prof. V.G. Lazarenkov Memory) (St. Petersburg Mining Univ., St. Petersburg, 2016), pp. 66–70 [in Russian].
F. Dawood, B. M. Leonard, and R. E. Schaak, Chem. Mater. 19 (18), 4545–4550 (2007).
N. K. Beck, B. Steiger, G. G. Scherer, and A. Wokaun, Fuel Cells 6 (1), 26–30 (2006).
Funding
This study was carried out as a part of a State Assignment, project no. 121041500220-0, of the Institute of Geology of Ore Deposits, Petrography, Mineralogy, and Geochemistry, Russian Academy of Sciences. The synthesis was supported by a Grant of the President of the Russian Federation for state support of leading scientific schools of the Russian Federation, project no. NSh-2394.2022.1.5. Thermoradiography was conducted on the equipment of the Center for Collective Use, Institute of Physical Chemistry and Electrochemistry, Russian Academy of Sciences. Analytical data of electron microprobe analysis were obtained at the Laboratory for Local Methods of the Study of Matter (Department of Petrology and Volcanology, Faculty of Geology, Moscow State University) using a Superprobe JEOL JXA-8230 electron microprobe purchased with funds from the Moscow University Development Program.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
The authors declare that they have no conflicts of interest.
Additional information
Translated by A. Bobrov
Rights and permissions
About this article
Cite this article
Mezhuyeva, A.A., Karimova, O.V., Zinovieva, N.G. et al. Thermal Transformation of a Synthetic Analogue of Insizwaite PtBi2 on Air. Dokl. Earth Sc. 506, 740–748 (2022). https://doi.org/10.1134/S1028334X22600529
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S1028334X22600529