Abstract
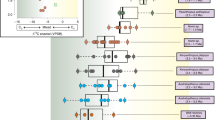
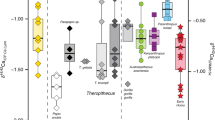
Stable isotope analyses are frequently used to study trophic interactions, diet, and community processes, but they have seldom been applied to investigate the trophic niche structure of entire clades. In this paper, we assess stable isotopes information in a phylogenetic context to evaluate trophic evolution across the phylogeny of a diversified group of Neotropical cricetid rodents. A total of 139 hair samples of 47 species of Akodontini rodents were collected from five museum mammal collections and submitted to stable isotope analyses for δ13C and δ15N values. The resulting isotopic values were compared among the four main clades within the tribe. The phylogenetic signal of isotope values was estimated using a phylogenetic tree of Akodontini. Our results corroborate previous impressions that, in general, akodontines include more animal matter in their diet than other Neotropical rodents, but the lack of information for some species precludes more specific inferences. Some species appear to have relatively restricted niches, but the large variance observed in other species may be related to dietary and habitat differences related to ecological factors throughout the distribution of wide-ranging species. We found low phylogenetic signal for δ13C and δ15N values, suggesting that different regions within the isotopic niche space were occupied independently many times throughout akodontine evolutionary history. The δ13C/δ15N bi-plot indicates that the four main lineages occupy the trophic niche space in similar ways, although differing in trophic diversity. Our results represent new ecological information and an approach that can be useful in studying the evolution of trophic niches, and highlight the importance of museum specimen-based research for evolutionary ecology studies.




Similar content being viewed by others
References
Ambrose SH (1991) Effects of diet, climate and physiology on nitrogen isotope abundances in terrestrial food webs. J Archaeol Sci 18(3):293–317
Amundson R, Austin AT, Schuur EA, Yoo K, Matzek V, Kendall C, Uebersax A, Brenner D, Baisden WT (2003) Global patterns of the isotopic composition of soil and plant nitrogen. Glob Biogeochem Cycles 17(1):1031
Baltensperger AP, Huettmann F, Hagelin JC, Welker JM (2015) Quantifying trophic niche spaces of small mammals using stable isotopes (δ15N and δ13C) at two scales across Alaska. Can J Zool 93(7):579–588
Barlow JC (1969) Observations on the biology of rodents in Uruguay. Life Sci Contrib R Ont Mus 75:1–59
Bate GC (1981) Nitrogen cycling in savanna ecosystems. Ecol Bull 33:463–475
Bearhop S, Waldron S, Votier SC, Furness RW (2002) Factors that influence assimilation rates and fractionation of nitrogen and carbon stable isotopes in avian blood and feathers. Physiol Biochem Zool 75(5):451–458
Bearhop S, Adams CE, Waldron S, Fuller RA, MacLeod H (2004) Determining trophic niche width: a novel approach using stable isotope analysis. J Anim Ecol 73(5):1007–1012
Becker EL, Cordes EE, Macko SA, Lee RW, Fisher CR (2013) Using stable isotope compositions of animal tissues to infer trophic interactions in Gulf of Mexico lower slope seep communities. PLoS ONE 8(12):e74459
Ben-David M, Flaherty EA (2012) Stable isotopes in mammalian research: a beginner’s guide. J Mammal 93(2):312–328
Bender MM (1971) Variations in the 13C/12C ratios of plants in relation to the pathway of carbon dioxide fixation. Phytochemistry 10:1239–1244
Bezerra AM, Pardiñas UFJ (2016) Kunsia tomentosus (Rodentia: Cricetidae). Mamm Species 48:1–9
Blomberg SP, Garland T, Ives AR (2003) Testing for phylogenetic signal in comparative data: behavioral traits are more labile. Evolution 57:717–745
Bocherens H, Fizet M, Mariotti A (1994) Diet, physiology and ecology of fossil mammals as inferred from stable carbon and nitrogen isotope biogeochemistry: implications for Pleistocene bears. Palaeogeogr Palaeoclimatol Palaeoecol 107(3–4):213–225
Boecklen WJ, Yarnes CT, Cook BA, James AC (2011) On the use of stable isotopes in trophic ecology. Annu Rev Ecol Evol Syst 42:411–440
Casey MM, Post DM (2011) The problem of isotopic baseline: reconstructing the diet and trophic position of fossil animals. Earth Sci Rev 106(1–2):131–148
Cerling TE, Ehleringer JR (2000) Welcome to the C4 world. In: Gastaldo RA, DiMichele WA (eds) Phanerozoic terrestrial ecosystems, the paleontological society papers. Yale University Press, New Haven, pp 273–286
Cerqueira R, Santori RT, Gentile R, Guapyassu SMS (2003) Micrographical ecological differences between two populations of Akodon cursor (Rodentia, Sigmodontinae) in a Brazilian Restinga. J Adv Zool 24:46–52
Chen S, Bai Y, Lin G, Han X (2005) Variations in life-form composition and foliar carbon isotope discrimination among eight plant communities under different soil moisture conditions in the Xilin River Basin, Inner Mongolia, China. Ecol Res 20(2):167–176
Chiaradia A, Ramírez F, Forero MG, Hobson KA (2016) Stable Isotopes (δ13C, δ15N) combined with conventional dietary approaches reveal plasticity in central-place foraging behavior of little penguins Eudyptula minor. Front Ecol Evol 3:00154
Crowley BE, Godfrey LR, Irwin MT (2011) A glance to the past: subfossils, stable isotopes, seed dispersal, and lemur species loss in southern Madagascar. Am J Primatol 73(1):25–37
D’Elía G, Pardiñas UFJ (2015) Tribe Akodontini Vorontsov 1959. In: Patton JL, Pardiñas UFJ, D’Elía G (eds) Mammals of South America, vol 2. Chicago University Press, Chicago, pp 140–144
Dalerum F, Angerbjörn A (2005) Resolving temporal variation in vertebrate diets using naturally occurring stable isotopes. Oecologia 144(4):647–658
Dammhahn M, Soarimalala V, Goodman SM (2012) Trophic niche differentiation and microhabitat utilization in a species-rich montane forest small mammal community of eastern Madagascar. Biotropica 45(1):111–118
Davis M, Pineda-Munoz S (2016) The temporal scale of diet and dietary proxies. Ecol Evol 6(6):1883–1897
DeNiro MJ (1987) Stable isotopy and archaeology. Am Sci 75(2):182–191
DeNiro MJ, Epstein S (1978) Influence of diet on the distribution of carbon isotopes in animals. Geochim Cosmochim Acta 42(5):495–506
DeNiro MJ, Epstein S (1981) Influence of diet on the distribution of nitrogen isotopes in animals. Geochim Cosmochim Acta 45(3):341–351
Diório DG (2014) Análise da espécie Bibimys labiosus (Winge 1887) (Rodentia, Sigmodontinae) ao longo da sua distribuição geográfica no Brasil. Dissertation, Universidade Federal de Ouro Preto
Dittel JW, Lambert TD, Adler GH (2015) Seed dispersal by rodents in a lowland forest in central Panama. J Trop Ecol 31(5):403–412
Dobson FS, Oli MK (2007) Fast and slow life histories of mammals. Ecoscience 14:292–299
Ellis BA, Mills JN, Glass GE, McKee KT Jr, Enria DA, Childs JE (1998) Dietary habits of the common rodents in an agroecosystem in Argentina. J Mammal 79:1203–1220
Emmons LH, Patton JL (2012) Taxonomic revision of Bolivian Juscelinomys (Rodentia, Cricetidae) with notes on morphology and ecology. Mammalia 76:285–294
Feranec RS, Hadly EA, Paytan A (2009) Stable isotopes reveal seasonal competition for resources between late Pleistocene bison (Bison) and horse (Equus) from Rancho La Brea, southern California. Palaeogeogr Palaeoclimatol Palaeoecol 271(1–2):153–160
Fox-Dobbs K, Stidham TA, Bowen GJ, Emslie SD, Koch PL (2006) Dietary controls on extinction versus survival among avian megafauna in the late Pleistocene. Geology 34(8):685–688
Fox-Dobbs K, Leonard JA, Koch PL (2008) Pleistocene megafauna from eastern Beringia: paleoecological and paleoenvironmental interpretations of stable carbon and nitrogen isotope and radiocarbon records. Palaeogeogr Palaeoclimatol Palaeoecol 261(1–2):30–46
Fry B (ed) (2006) Stable isotope ecology. Springer, New York
Galetti M, Rodarte RR, Neves CL, Moreira M, Costa-Pereira R (2016) Trophic niche differentiation in rodents and marsupials revealed by stable isotopes. PLoS ONE 11(4):e0152494
Gannes LZ, Del Rio CM, Koch PL (1998) Natural abundance variations in stable isotopes and their potential uses in animal physiological ecology. Comput Biochem Phys A 119(3):725–737
Geise L, Bergallo HG, Esberárd CEI, Rocha CFD, Van Sluys M (2008) The karyotype of Blarinomys breviceps (Mammalia: Rodentia: Cricetidae) with comments on its morphology and some ecological notes. Zootaxa 1907:47–60
Geise L, Paresque R, Sebastão H, Shirai LT, Astúa D, Marroig G (2010) Non-volant mammals, Parque Nacional do Catimbau, Vale do Catimbau, Buíque, Pernambuco State, Brazil, with karyologic data. Check List 6:180–186
Glanz WE (1984) Ecological relationships of two species of Akodon in central Chile. J Mammal 65(3):433–441
Granhall U (1981) Biological nitrogen fixation in relation to environmental factors and functioning of natural ecosystems. In: Clark FE, Rosswall T (eds) Terrestrial nitrogen cycles. Ecological bulletins, vol 33. Swedish Natural Science Research Council, Stockholm, pp 131–144
Hammer Ø, Harper DAT, Ryan PD (2001) PAST: paleontological statistics software package for education and data analysis. Palaeontol Electron 4:9
Hershkovitz P (1966) South American swamp and fossorial rats of the scapteromyine group (Cricetinae, Muridae) with comments on the glans penis in murid taxonomy. Z Säugetierkd 31:81–149
Hershkovitz P (1994) Description of a new species of South American hocicudo, or long-nose mouse, genus Oxymycterus (Sigmodontinae, Muroidea), with a critical review of the generic content. Fieldiana Zool 79:1–43
Hilderbrand GV, Farley SD, Robbins CT, Hanley TA, Titus K, Servheen C (1996) Use of stable isotopes to determine diets of living and extinct bears. Can J Zool 74(11):2080–2088
Hobson KA, Koehler G (2015) On the use of stable oxygen isotope (δ18O) measurements for tracking avian movements in North America. Ecol Evol 5(3):799–806
Huiman MCN (2008) Dieta y morfología del estómago en roedores de los bosques montanos del departamento Huánuco, Perú. Dissertation, Universidad Nacional Mayor de San Marcos
Jackson AL, Inger R, Parnell AC, Bearhop S (2011) Comparing isotopic niche widths among and within communities: SIBER—stable isotope Bayesian ellipses in R. J Anim Ecol 80(3):595–602
Jonsson P, Koskela E, Mappes T (2000) Does risk of predation by mammalian predators affect the spacing behaviour of rodents? Two large-scale experiments. Oecologia 122(4):487–492
Kamilar JM, Cooper N (2013) Phylogenetic signal in primate behaviour, ecology and life history. Philos Trans R Soc Lond B 368(1618):20120341
Kelly JF (2000) Stable isotopes of carbon and nitrogen in the study of avian and mammalian trophic ecology. Can J Zool 78(1):1–27
Knight JD, Thies JE, Singleton PW, Van Kessel C (1995) Carbon isotope composition of N2-fixing and N-fertilized legumes along an elevational gradient. Plant Soil 177(1):101–109
Kohn MJ (2010) Carbon isotope compositions of terrestrial C3 plants as indicators of (paleo) ecology and (paleo) climate. Proc Natl Acad Sci 107(46):19691–19695
Kotler BP, Brown JS, Mitchell WA (1994) The role of predation in shaping the behavior, morphology and community organization of desert rodents. Aust J Zool 42(4):449–466
Layman CA, Araujo MS, Boucek R, Hammerschlag-Peyer CM, Harrison E, Jud ZR, Matich P, Rosenblatt AE, Vaudo JJ, Yeager LA, Post DM (2012) Applying stable isotopes to examine food-web structure: an overview of analytical tools. Biol Rev 87(3):545–562
Losos JB (2008) Phylogenetic niche conservatism, phylogenetic signal and the relationship between phylogenetic relatedness and ecological similarity among species. Ecol Lett 11(10):995–1003
MacLaren JA, Hulbert RC, Wallace SC, Nauwelaerts S (2018) A morphometric analysis of the forelimb in the genus Tapirus (Perissodactyla: Tapiridae) reveals influences of habitat, phylogeny and size through time and across geographical space. Zool J Linn Soc 184:499–515
Maestri R, Monteiro LR, Fornel R, Upham NS, Patterson BD, de Freitas TRO (2017) The ecology of a continental evolutionary radiation: Is the radiation of sigmodontine rodents adaptive? Evolution 71(3):610–632
Marshall JD, Brooks JR, Lajtha K (2007) Sources of variation in the stable isotopic composition of plants. In: Michener R, Lajtha K (eds) Stable isotopes in ecology and environmental science. Blackwell Scientific, London, pp 22–60
Martínez del Rio C, Wolf N, Carleton SA, Gannes LZ (2009) Isotopic ecology ten years after a call for more laboratory experiments. Biol Rev Camb Philos Soc 84:91–111
Merceron G, Zazzo A, Spassov N, Geraads D, Kovachev D (2006) Bovid paleoecology and paleoenvironments from the Late Miocene of Bulgaria: evidence from dental microwear and stable isotopes. Palaeogeogr Palaeoclimatol Palaeoecol 241(3–4):637–654
Meserve PL, Lang BK, Patterson BD (1988) Trophic relationships of small mammals in a Chilean temperate rainforest. J Mammal 69(4):721–730
Miller GH, Fogel ML, Magee JW, Gagan MK, Clarke SJ, Johnson BJ (2005) Ecosystem collapse in Pleistocene Australia and a human role in megafaunal extinction. Science 309(5732):287–290
Miller JF, Millar JS, Longstaffe FJ (2008) Carbon-and nitrogen-isotope tissue–diet discrimination and turnover rates in deer mice, Peromyscus maniculatus. Can J Zool 86(7):685–691
Newsome SD, Martinez del Rio C, Bearhop S, Phillips DL (2007) A niche for isotopic ecology. Front Ecol Environ 5(8):429–436
Nielsen JM, Clare EL, Hayden B, Brett MT, Kratina P (2017) Diet tracing in ecology: method comparison and selection. Methods Ecol Evol 9(2):278–291
O’Connell TC, Hedges REM (1999) Investigations into the effect of diet on modern human hair isotopic values. Am J Phys Anthropol 108:409–425
Ofukany AF, Wassenaar LI, Bond AL, Hobson KA (2014) Defining fish community structure in Lake Winnipeg using stable isotopes (δ13C, δ15N, δ34S): implications for monitoring ecological responses and trophodynamics of mercury and other trace elements. Sci Total Environ 497:239–249
O’Leary MH (1988) Carbon isotopes in photosynthesis. Bioscience 38(5):328–336
O’Leary MH, Madhavan S, Paneth P (1992) Physical and chemical basis of carbon isotope fractionation in plants. Plant Cell Environ 15(9):1099–1104
Pardiñas UFJ, Teta P, Alvarado-Serrano D, Geise L, Jayat JP, Ortiz PE, Gonçalves PR, D’Elia G (2015) Genus Akodon Meyen. In: Patton JL, Pardiñas UFJ, D’Elía G (eds) Mammals of South America, vol 2. Chicago University Press, Chicago, pp 144–204
Pardiñas UFJ, Geise L, Ventura K, Lessa G (2016a) A new genus for Habrothrix angustidens and Akodon serrensis (Rodentia, Cricetidae): again paleontology meets neontology in the legacy of Lund. Mastozool Neotrop 23:93–115
Pardiñas UFJ, Patterson BD, D’ElIa G, Teta P (2016b) Bibimys labiosus (errata version published in 2017). The IUCN Red List of Threatened Species 2016:e.T2802A115063837. http://dx.doi.org/10.2305/IUCN.UK.2016-3.RLTS.T2802A22356418.en. Accessed Jan 2019
Pardiñas UFJ, Voglino D, Galliari CA (2017) Miscellany on Bibimys (Rodentia, Sigmodontinae), a unique akodontine cricetid. Mastozool Neotrop 24(1):241–250
Parnell A, Jackson A (2013) siar: stable isotope analysis in R. R package version 4.2
Pauli JN, Newsome SD, Cook JA, Harrod C, Steffan SA, Baker CJ, Ben-David M, Bloom D, Bowen GJ, Cerling TE, Cicero C (2017) Opinion: why we need a centralized repository for isotopic data. Proc Natl Acad Sci 114(12):2997–3001
Peterson BJ, Fry B (1987) Stable isotopes in ecosystem studies. Annu Rev Ecol Syst 18(1):293–320
Pineda-Munoz S, Lazagabaster IA, Alroy J, Evans AR (2017) Inferring diet from dental morphology in terrestrial mammals. Methods Ecol Evol 8(4):481–491
Pinotti BT, Naxara L, Pardini R (2011) Diet and food selection by small mammals in an old-growth Atlantic forest of south-eastern Brazil. Stud Neotrop Fauna E 46(1):1–9
Pizzimenti JJ, De Salle ROB (1980) Dietary and morphometric variation in some Peruvian rodent communities: the effect of feeding strategy on evolution. Biol J Linn Soc 13(4):263–285
Post DM (2002) Using stable isotopes to estimate trophic position: models, methods, and assumptions. Ecology 83(3):703–718
R Core Team (2013) R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna
Read AF, Harvey PH (1989) Life history differences among the eutherian radiations. J Zool 219(2):329–353
Reichman OJ (1975) Relation of desert rodent diets to available resources. J Mammal 56(4):731–751
Reig OA (1972) The evolutionary history of the South American cricetid rodents. Dissertation, University of London
Reig OA (1980) A new fossil genus of South American cricetid rodents allied to Wiedomys, with an assessment of the Sigmodontinae. J Zool 192(2):257–281
Revell LJ (2012) Phytools: an R package for phylogenetic comparative biology (and other things). Methods Ecol Evol 3:217–223
Samuels JX (2009) Cranial morphology and dietary habits of rodents. Zool J Linn Soc Lond 156(4):864–888
Schmitt CJ, Cook JA, Zamudio KR, Edwards SV (2018) Museum specimens of terrestrial vertebrates are sensitive indicators of environmental change in the Anthropocene. Philos Trans R Soc Lond B 374:20170387
Secord R, Wing SL, Chew A (2008) Stable isotopes in early Eocene mammals as indicators of forest canopy structure and resource partitioning. Paleobiology 34(2):282–300
Shearer G, Kohl DH (1986) N2-fixation in field settings: estimations based on natural 15N abundance. Funct Plant Biol 13(6):699–756
Smiley TM, Cotton JM, Badgley C, Cerling TE (2015) Small-mammal isotope ecology tracks climate and vegetation gradients across western North America. Oikos 125:1100–1109
Solari S (2007) Trophic relationships within a highland rodent assemblage from Manu National Park, Cusco, Peru. In: Kelt DA, Lessa EP, Salazar-Bravo J, Patton JL (eds) The quintessential naturalist: honoring the life and legacy of Oliver P. Pearson. University of California Press, Berkeley, pp 225–240
Sparks JP, Ehleringer JR (1997) Leaf carbon isotope discrimination and nitrogen content for riparian trees along elevational transects. Oecologia 109(3):362–367
Sponheimer M, Robinson T, Ayliffe L, Roeder B, Hammer J, Passey B, West A, Cerling T, Dearing D, Ehleringer J (2003) Nitrogen isotopes in mammalian herbivores: hair δ15N values from a controlled feeding study. Int J Osteoarchaeol 13(1–2):80–87
Steppan SJ, Schenk JJ (2017) Muroid rodent phylogenetics: 900-species tree reveals increasing diversification rates. PLoS ONE 12(8):e0183070
Sulzman EW (2007) Stable isotope chemistry and measurement: a primer. In: Michener R, Lajtha K (eds) Stable isotopes in ecology and environmental science. Blackwell Scientific, London, pp 1–21
Swap RJ, Aranibar JN, Dowty PR, Gilhooly WP, Macko SA (2004) Natural abundance of C-13 and N-15 in C-3 and C-4 vegetation of southern Africa: patterns and implications. Glob Change Biol 10(3):350–358
Talamoni SA, Couto D, Júnior DAC, Diniz FM (2008) Diet of some species of neotropical small mammals. Mamm Biol 73(5):337–341
Teta P, Cueto G, Suarez O (2007) New data on morphology and natural history of Deltamys kempi Thomas, 1919 (Cricetidae, Sigmodontinae) from central-eastern Argentina. Zootaxa 1665(1):43–51
Thomas O (1918) On small mammals from Salta and Jujuy collected by Mr. E. Budin. Ann Mag Nat Hist ser 9:186–193
Tieszen LL, Boutton TW, Tesdahl KG, Slade NA (1983) Fractionation and turnover of stable carbon isotopes in animal tissues: implications for δ13C analysis of diet. Oecologia 57(1–2):32–37
Turner TF, Collyer ML, Krabbenhoft TJ (2010) A general hypothesis-testing framework for stable isotope ratios in ecological studies. Ecology 91(8):2227–2233
van Bergen E, Barlow HS, Brattström O, Griffiths H, Kodandaramaiah U, Osborne CP, Brakefield PM (2016) The stable isotope ecology of mycalesine butterflies: implications for plant–insect co-evolution. Funct Ecol 30(12):1936–1946
Van der Merwe NJ (1982) Carbon isotopes, photosynthesis, and archaeology: different pathways of photosynthesis cause characteristic changes in carbon isotope ratios that make possible the study of prehistoric human diets. Am Sci 70(6):596–606
Vander Wall SB (2003) Effects of seed size of wind-dispersed pines (Pinus) on secondary seed dispersal and the caching behavior of rodents. Oikos 100(1):25–34
Vander Zanden HB, Tucker AD, Hart KM, Lamont MM, Fujisaki I, Addison DS, Mansfield KL, Phillips KF, Wunder MB, Bowen GJ, Pajuelo M (2015) Determining origin in a migratory marine vertebrate: a novel method to integrate stable isotopes and satellite tracking. Ecol Appl 25(2):320–335
Vargas J, Flores P, Martínez J (2007) Pequeños mamíferos en dos áreas protegidas de la vertiente oriental boliviana, considerando la variación altitudinal y la formación vegetacional. Revista Virtual Redesma. http://revistavirtual.redesma.org/vol2/pdf/ambiental/mamiferos.pdf. Accessed Dec 2018
Vogel JC (1993) Variability of carbon isotope fractionation during photosynthesis. In: Ehleringer JR, Hall AE, Farquhar GD (eds) Stable isotopes and plant carbon–water relations. Academic Press, San Diego, pp 29–38
Williams SH, Kay RF (2001) A comparative test of adaptive explanations for hypsodonty in ungulates and rodents. J Mamm Evol 8(3):207–229
Zevallos C (2014) Dieta de roedores sigmodontinos (Rodentia: Cricetidae) en los bosques montanos del valle del Río Holpas, Ayacucho-Perú. Dissertation, Universidad Nacional Mayor de San Marcos
Acknowledgements
We thank Robert Voss, Darrin Lunde, Louise Emmons and Claudia Costa who kindly allowed the sampling of specimens under their care for the stable isotope analysis. We gratefully acknowledge Isabel Distefano and Kevin Feldheim from the Field Museum’s Pritzker Laboratory for Molecular Systematics and Evolution, and Jennifer Melo de Andrade and Teofânia Dutra Amorim from the Centro de Pesquisas Hidráulicas of Universidade Federal de Minas Gerais for the logistical assistance with the preparation of the hair samples. We thank Chandelle Macdonald and the Stable Isotope Facility staff of the University of Wyoming for performing the stable isotope analysis of the samples; and Adam Ferguson and John Phelps from the Field Museum of Natural History for help with the sampling process and shipping. Regan Dunn, Raisa Rodarte and Raul Costa provided useful information on stable isotope analyses on preliminary stages of this work. We are grateful for useful comments from two anonymous reviewers and from the Associate Editor and Editor-in-Chief, that greatly improved the manuscript. The dispatch of the samples from Brazil was authorized according to registration A06DC31 of Sisgen. Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES) gave R.V.M. financial support through regular (Finance Code 0001) and PDSE (88881.133833/2016-1) fellowships.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflicts of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Missagia, R.V., Patterson, B.D. & Perini, F.A. Stable isotope signatures and the trophic diversification of akodontine rodents. Evol Ecol 33, 855–872 (2019). https://doi.org/10.1007/s10682-019-10009-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10682-019-10009-0