Abstract
Diet is a major driver of hominin evolution, but most of the geochemical evidence relies on carbon isotopes (δ13C). Here, we report enamel stable calcium isotope (δ44/42Ca) values against δ13C values for several hominins and co-existing primates in the Turkana Basin area, circa 4 to 2 Ma. Australopithecus anamensis clusters with mammal browsers, Kenyanthropus platyops is distinct from A. anamensis in foraging into more open environments and the coexisting Theropithecus brumpti encompasses both the grazer and omnivore/carnivore domains. Early Homo is remarkable for its wide distribution in δ44/42Ca values, possibly reflecting omnivorous and opportunistic preferences. Paranthropus boisei is uniquely distributed in the δ13C versus δ44/42Ca iso-space being distinct from all other hominins from the Turkana Basin area as well as from the co-existing Theropithecus oswaldi. Several hypotheses are explored to discuss the unique δ44/42Ca values of Paranthropus boisei including significant differences observed with δ44/42Ca values recently reported for P. robustus from South Africa, questioning the monophyly of this genus.
Similar content being viewed by others
Introduction
Non-traditional stable isotopes are providing new avenues of research for exploring the ecology, physiology, and dietary preferences of extinct organisms, including our own lineage. Current evidence for early hominin diet relies on comparative osteology, dental tribology, and geochemistry1,2,3,4,5,6,7,8,9,10. But in the latter case, collagen nitrogen and its isotopes, a well-used trophic level proxy, are rarely preserved in such ancient (i.e., >1 Ma) contexts. While non-traditional isotopes emerge as a new toolkit to paleodietary inference11,12,13,14,15, a great deal of the geochemical evidence for early hominin diets relies on carbon isotopes1,2,3,4,5,6,7,8,9 and to a lesser extent on trace element concentrations preserved in mineralized tissues such as fossil bone or teeth16,17,18,19. The carbon isotopic composition of bioapatite reflects the photosynthetic pathway (i.e., C3, C4, or CAM) of the ultimate plant source and has been useful for reconstructing past vegetation evolution20 and exploring the feeding ecology of African hominins and associated faunas. African hominins cover the whole C3–C4 spectrum. For example, in East Africa, early australopithecines derived most of their food from C3 sources1,4,6 whereas more recent forms such as Paranthropus boisei almost exclusively relied on C4 sources3,5. However, significant proportions of C4 sources are recorded in some early forms such as Australopithecus afarensis or Kenyanthropus platyops1,2,7. In Central Africa, Australopithecus bahrelghazali represents another example of an early hominin that was sourcing its food in a C4 environment8. Another significant result of carbon isotope studies is the marked difference in δ13C values between megadont australopithecines, providing evidence that the South African Paranthropus robustus and the East African Paranthropus boisei were exploiting different resources9,17. Such results were independently corroborated with microwear texture10,21. Nonetheless, other proxies are needed to infer hominin diet beyond the ultimate plant source. Here, we investigate resource use in Turkana Basin hominins from Kenya using calcium and carbon isotopes and discuss their relevance for inferring the evolution of dietary habits in the hominin lineage
Seminal studies on calcium isotopes in vertebrates22 recognized a decrease in the 44Ca/42Ca ratio with increasing trophic level. Subsequent studies supported this trophic level effect, notably in the marine realm23,24, but also raised awareness on individual physiological variability influencing Ca isotope variability25,26 or on the insensitivity of Ca isotope ratios to distinguish between primary and secondary consumers27. However, the constant δ44/42Ca offset of about −0.6‰ from dietary Ca to bone22,28 supports the idea of a propagation-related calcium isotope fractionation from one trophic level to another. This pattern reflects a shared physiological feature of all vertebrates including terrestrial and aquatic mammals, as measured in six different species of mammals including foregut and hindgut digestive physiologies (as reviewed in14). Moreover, this trophic level prediction recently found support as measured in a variety of modern and extinct terrestrial faunas29,30. Yet, disentangling dietary sources remains challenging because dietary items possess highly variable calcium concentrations and calcium isotopic compositions as exemplified by plants (as compiled in14,29), bone31 or milk28,32. Notably, this last source is 44Ca-depleted28 and analyzing tissues mineralizing during the pre- to postnatal transition has proved useful to study nursing in modern and fossil hominins32,33. Therefore, the isotopic composition of a consumer’s tissue is controlled by the mass-weighted isotopic average of all the dietary sources and in our present case, we do not include data from early forming teeth (e.g., first molars). In other words, whether 44Ca-enriched or 44Ca-depleted, a narrow range of calcium isotopic composition in a consumer tissue may reflect a specialized diet (e.g., late forming teeth of Gorilla, see below and Supplementary Data 1) whereas a scattered distribution should reflect a more complex interplay between resource diversity, isotopic variation in resources, and differential consumption of resources by individuals within a group. As explained above, we favor the hypothesis of a Ca isotope variability primarily related to diet but other aspects such as species-dependent physiologic parameters25,26 will have to be further explored in future calcium isotope mammal studies. Therefore, in mammals, calcium isotopes can be used to monitor nursing in early formed teeth28,32; later formed teeth can provide information on the intake of calcium (Ca) from adult dietary sources29.
Here, we report Ca isotope values for several early hominin species and associated nonhuman primates from the Turkana Basin area. We infer a unique trophic ecology and/or physiology for Paranthropus boisei according to its distinct isotopic distribution.
Results & discussion
Ca isotopic variability in modern Gorilla teeth
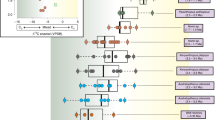
Teeth belonging to four modern western lowland gorilla (Gorilla gorilla gorilla) individuals from La Lopé National Park, Gabon, analyzed for Ca isotopes33, are presented here as a comparison with other hominin and non-hominin primates (Fig. 1 and Supplementary Data 1). Modern gorillas represent an interesting case study so as to test whether a monotonous herbivorous diet is reflected upon the Ca isotopic composition of bioapatite. Two of the sampled teeth were formed early and include an incisor (G72) with a distinctly low δ44/42Ca value as well as a first molar (G39) with a surprisingly high δ44/42Ca value, being indistinct from the two other late forming gorilla teeth (second and third molars) available (Supplementary Data 1). The first molar starts mineralization earlier than the incisor (i.e., before birth) and completes mineralization before the third year (within the fourth year for the incisor)34. Nevertheless, it should be stressed that tooth enamel from that first molar was sampled for the entire height of the crown, thus mixing enamel formed in utero with enamel formed during or after completion of weaning. Because such teeth mineralize until 3 year of age after birth35, weaning and adult food items other than breast milk (in this case plant material) would have contributed to the high δ44/42Ca values observed here36. It should also be added that dental development is genetically controlled and the huge genetic diversity reported in western lowland gorillas37 suggests that a wider spectrum of mineralization patterns exists beyond the published ages reconstructed from histological studies. Therefore, that the first molar (G72) did mineralize later than expected from the dental development models would not be surprising. The two late forming teeth (G40 and G41) display 44Ca-enriched compositions and are interpreted as reflecting the post-weaning period of tooth formation during which the individuals were taking their Ca from adult food33. A detailed survey of Ca isotope variability in gorilla teeth and other modern primates will certainly bring interesting results to discuss physiological versus dietary influence on Ca isotopic fractionation processes, as currently investigated in humans26,32,38.
Note the 44Ca-enriched isotope values of Paranthropus boisei in comparison to other groups. The boxes represent the first and third quartiles with the medians as horizontal lines. The lower and upper whiskers represent 1.5 * the interquartile range (numbers of biologically independent samples per group: n = 9 for Colobini; n = 5 for Papio anubis; n = 4 for Parapapio sp.; n = 2 for Theropithecus gelada; n = 7 for Theropithecus brumpti; n = 8 for Theropithecus oswaldi; n = 4 for Gorilla gorilla gorilla; n = 8 for Australopithecus anamensis; n = 5 for Kenyanthropus platyops; n = 8 for Paranthropus boisei; n = 13 for early Homo). Welch’s one-way ANOVA and Kruskal–Wallis tests show significant differences of average means (Welch’s one-way ANOVA on all groups except the two T. gelada individuals: 10 groups with a total of 71 biologically independent individual samples, p value < 10−4, F = 11.7, df = 9; Kruskal–Wallis on all groups: 11 groups with a total of 73 biologically independent individual samples, p value = 0.0005, df = 10). δ44/42Ca values are expressed both against ICP Ca Lyon (left) and SRM915a (right). Source data are provided as a Source Data file.
Ca isotopic variability in non-hominin fossil primates
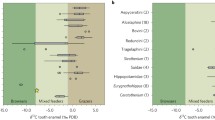
Our stable calcium isotope values for various hominins and co-existing primates from the Turkana Basin area (n = 69) range between −0.69‰ and −1.88‰ (Figs. 1 and 2; Supplementary Data 1). Recently published calcium isotope data for coeval large mammals were shown to preserve their pristine composition, as discussed against elemental concentrations of diagenetic origin of the same samples29 (see also Methods). This dataset presents a similar ordering between modern and fossil faunas but also some differences for certain taxa such as suids and saber-tooth cats, interpreted to have had changing dietary ecologies through time29.
The colored areas represent the domains for previously analyzed extant terrestrial mammal enamel from the Turkana Basin area29 (n = 4 for leopards; n = 10 for lions and hyaenas; n = 24 for browsers; n = 18 for grazers). δ44/42Ca values are expressed both against ICP Ca Lyon (left) and SRM915a (right). The average 2SD of each Ca isotopic value is represented at the bottom left of each graph. Source data are provided as a Source Data file.
Papionines have often been considered as analogues to understand dietary preferences of early hominins1. The 44Ca-depleted isotope values of modern Papio anubis (Fig. 1) are to be considered under the light of the broad feeding preferences in cercopithecines39,40. In comparison, the more positive Ca isotope values of Parapapio sp. invite to consider that extinct taxa may not have been similar in their feeding preferences, although one must take into account that modern Papio anubis has a wide distribution in Africa today and our data cannot reflect such spatial variations. Modern geladas do intensively feed on high altitude C3 grasses41 and our δ44/42Ca values (Supplementary Data 1) for two modern grass-eating Theropithecus gelada (−1.40‰) are consistent with a grass-eating ecology. Grazers are 44Ca-depleted relative to leaf-eaters by about 0.3‰ and other modern grazers such as zebras, buffaloes, warthogs, and hippos also display comparably low calcium isotope values that overlap with carnivore values29 (Fig. 2). Morphological evidence shows grass-eating adaptations in extinct Theropithecus42; carbon isotopes confirm an early dietary adaptation to C4 grasses as an important component of their diet43. Here, δ44/42Ca values for T. brumpti (−1.36 ± 0.31‰, 1SD, n = 7) and T. oswaldi (−1.26 ± 0.27‰, 1SD, n = 8) are dispersed indicating the availability of a range of isotopically different calcium sources consumed in different proportions by different individuals in each species. Some of those values are not departing much from those of the modern T. gelada, and a grass-eating ecology is a probable dietary hypothesis for some individuals of both fossil species. Nevertheless, this does not exclude other food sources, for example as illustrated here with 44Ca-depleted values for several individuals of T. brumpti falling within the same range of values as those of large carnivores (Fig. 2b). T. brumpti was a large-sized theropithecine compared with T. oswaldi or to the extant T. gelada and contrary to those, T. brumpti was clearly foraging into mixed environments according to δ13C values. Given the wide range of δ44/42Ca values observed in T. brumpti, an omnivorous diet, sometime including bone, is plausible.
Ca isotopic variability in fossil hominins
Australopithecus anamensis and Kenyanthropus platyops could not be distinguished on the basis of calcium isotope values. K. platyops carbon isotope values clearly showed that it foraged in mixed C3-C4 environments whereas values for A. anamensis indicate a nearly pure-C3 diet1 similar to Ardipithecus ramidus4. Although early studies based on cranial morphology suggested tough/abrasive food items in the diet of A. anamensis, more recent studies based on microwear and carbon isotopes proposed a diet of soft plant items from the C3 environment1,44. Here, the distribution of calcium isotope values in A. anamensis (−1.18 ± 0.17‰, 1SD, n = 8) is not distinctly 44Ca-depleted and overlaps much of the modern and fossil East African browser domains (Fig. 2) and indicates that it was foraging on a variety of C3 resources, perhaps fruits or plants, as previously suggested from carbon isotopes1. Some individuals of A. anamensis may have spent some time in open habitats as indicated by positive δ18O values, clearly distinct from the 18O-depleted values of Parapapio sp. and colobines (Supplementary Fig. 1, Supplementary Data 1), suggesting a decoupling between food and habitat use.
The δ44/42Ca values in the early Homo group are dispersed (−1.36 ± 0.23‰, 1SD, n = 13) and may either indicate wide dietary flexibility, incorporating different sources with varied calcium isotopic values and concentrations, possibly including omnivorous or carnivorous preferences for the most 44Ca-depleted isotope values. In addition, such values may reflect a lack of taxonomic resolution within a diversified early Homo group in the Turkana Basin area containing Homo habilis, Homo rudolfensis, and Homo erectus45, and could be due to heterogeneous dietary habits within the genus Homo. Also notable is that early Homo (−1.36 ± 0.23‰, 1SD, n = 13) and Theropithecus oswaldi (−1.26 ± 0.27‰, n = 8) have similar calcium isotope values but not carbon isotope values.
The peculiar case of Paranthropus boisei
Paranthropus boisei displays the most 44Ca-enriched values of the dataset (−0.89 ± 0.11‰, 1SD, n = 8) being statistically different from early Homo (Welch’s t-test, p*** < 10−4), Theropithecus oswaldi (Welch’s t-test, p** = 0.006) and from Turkana mammal grazers (Welch’s t-test, p*** < 10−4). That P. boisei values do not overlap in a δ13C versus δ44/42Ca space with any other hominins and non-hominin grazers (Figs. 1 and 2; Supplementary Data 2) adds to the uniqueness of this taxon, as previously highlighted in the literature with morphological, microwear, or carbon isotopic studies.
P. boisei possesses a unique tooth morphology with thick enamel and flatten occlusal surfaces when worn, and dental microwear studies do suggest a diet of soft plant items21. If leaves appear to be a good dietary candidate, C4 leaves other than blades from grasses or sedges are not obviously identified in modern ecosystems. Theropithecus gelada cannot be considered a modern primate analogue for P. boisei, given their differences in δ44/42Ca values. A modern primate analogue for P. boisei that would feed on C4 vegetation other than grass or sedge does not exist. Gorillas could be viewed as a remote plant-eating analogue. The two late-forming modern western lowland gorilla teeth from La Lopé in Gabon display calcium isotope values around −1.05‰ (Supplementary Data 1)33, comparable with the values of P. boisei. Although those gorillas feed on fruits, leaves, and ants, these resources arise from C3, not C4 plants11. While dicot leaves and fruits are 44Ca-enriched, the only dicot leaves that follow the C4 photosynthetic pathway are represented by forbs, which would be found in relatively wet environments46 and could be a dietary candidate for P. boisei according to oxygen isotope composition of P. boisei teeth suggesting water dependency5. Conducting a calcium isotope survey of such plants but also of C4 sedges, especially in including plant parts that are known to fractionate differently47, would certainly add relevant data to better tackle this problem. On the other hand, we cannot exclude that both G. gorilla and P. boisei had physiological similarities that would lead to 44Ca/42Ca values that are enriched relative to other primates.
Whatever the exact dietary component or physiological specificity of P. boisei was, this species remains remarkable for exhibiting 44Ca-enriched tooth enamel within a narrow range of isotope values, which most likely reflect a specialized diet on items with low isotopic variability48 possibly associated to a physiology different than other primates. The range of calcium isotope values of P. boisei not only differs from other hominins of the Turkana Basin, it also differs from that of its South African congener P. robustus, which shows lower calcium isotope values33 (Fig. 3). Interestingly, P. robustus and A. africanus do not differ in δ13C values9, nor do they differ in δ44/42Ca values33 (Fig. 3). P. robustus was interpreted to have had a flexible diet together with the contemporaneous early Homo from South Africa9,49, a hypothesis that seems corroborated by the overlap of calcium isotope values of P. robustus with early Homo and A. africanus33. From our study as well as previous ones based on δ13C values1 or tooth microwear21, the emerging picture could be that of P. boisei in East Africa with a specialized diet versus P. robustus in South Africa with a flexible diet9,49. These results confirm discrepancies between diets but apparently shared masticatory function and weaken the case for Paranthropus monophyly based solely on dentognathic features50.
Data for grazers, browsers, and carnivores are from Martin et al.29 and Tacail et al.33. EA East Africa, SA South Africa. Values selected for representatives of the associated-fauna include only those values for organisms circa 2 Ma; for this reason, the browser category may appear underrepresented and the apparent difference may reflect specific dietary differences rather than differences in the substrate isotope baseline. Note that carnivore values in EA are dispersed as explained by a dataset that includes sabertooth felids. The boxes represent the first and third quartiles with the medians as horizontal lines. The lower and upper whiskers represent 1.5 * the interquartile range (numbers of biologically independent samples per group: n = 7 for South African carnivores; n = 10 for East African carnivores; n = 7 for South African browsers; n = 4 for East African browsers; n = 10 for South African grazers; n = 14 for East African grazers; n = 38 for Paranthropus robustus; n = 8 for Paranthropus boisei). δ44/42Ca values are expressed both against ICP Ca Lyon (left) and SRM915a (right). Source data are provided as a Source Data file.
In summary, calcium isotope ratios in a survey of hominin lineages in East Africa show that P. boisei has a unique 44Ca/42Ca ratio compared with other hominins including the South African P. robustus, all of which have similar ratios to each other but not to P. boisei. This calcium isotope difference could be due to differences in diet, or in physiology, and remains a unique character of P. boisei compared with other hominins.
Methods
Fossil samples
Fossil samples were available from the collections of the National Museums of Kenya and the Turkana Basin Institute. Those fossil samples are part of a paleoecology project reported in Cerling et al.51. Powdered enamel was collected using a low-speed dental drill along the broken surfaces of tooth enamel. Each sample represents an average of the crown height in order to provide enough material for the published carbon isotope analyses initially envisioned. 100–200 µg of powder per sample was used for Ca isotope purification.
Modern samples
Two geladas (Theropithecus gelada) individuals were made available from the historical collections of the Musée des Confluences in Lyon, France (MHNL). The precise Ethiopian origin of the specimens is not provided on the labels. Specimen MHNL 50001812 is a male individual and was donated to the Museum by Claudius Côte on the 13th of august 1929. Specimen MHNL 50001729 is a male individual from Abyssinia and was bought from Mr. Siepi, then a naturalist in Marseille on the 3rd of march 1925.
Sample chemistry
The chemical processing of the samples follows the method described in details in Martin et al.29 and Tacail et al33,52. Briefly, enamel samples were dissolved in 300 μl of suprapure 1 M HCl acid and were subsequently processed through AG50X-W12 cation exchange resin in 1 M HCl medium to dispose of sample matrix. Ca and Sr fractions were collected in 6 M HCl medium. Ca fractions were then separated from Sr by loading samples onto columns filled with Sr-specific resin (Eichrom Sr-Spec) in suprapure 2 M HNO3 medium. Blanks for the whole procedure did not exceed 100 ng Ca.
Isotopic measurements
Calcium isotope abundance ratios (44Ca/42Ca and 43Ca/42Ca) were measured using a multi-collector ICP-MS (MC-ICP-MS, Neptune Plus, Thermo). After purification, Ca samples were dissolved in ultrapure 0.05 M HNO3 and Ca concentration was set at 2 ppm for all samples and standards. All Ca isotope compositions are expressed using the ‘delta’ notation defined as follows for the 44Ca/42Ca ratio:
where (44Ca/42Ca)sample and (44Ca/42Ca)ICP Ca Lyon are the Ca isotope abundance ratios measured in sample and ICP Ca Lyon reference standard, respectively. The ICP Ca Lyon standard, used as a bracketing standard, is a Specpure Ca plasma standard solution (Alfa Aesar)12,24,30,32,33,52,53,54,55. All newly reported samples were measured as part of the same batches as the samples reported in Martin et al.29, i.e., in June and August 2016 and in June and July 2017. A single concentrated solution of NIST SRM 1486 was repeatedly purified and measured in the same batches as the samples to control for accuracy. In order to ease comparison of our dataset with other Ca isotope studies from the literature, all values obtained in this study are also expressed relative to SRM915a standard (Supplementary Data 1) using the constant difference of −0.518 ± 0.025‰ (2SD) as calibrated by analyzing four international standards previously reported against SRM915a and repeatedly measured against ICP Ca Lyon (see Supplementary Information in Martin et al.29). All statistical tests on the Ca isotope data were performed with the R “stats” package56.
Quality
Samples were randomly measured during the ICP-MS sessions that served to build the dataset published on the modern and fossil Turkana fauna in Martin et al.29. All samples fall on a calcium mass fractionation line with a slope of 0.519 ± 0.018‰ (2SE) (Supplementary Fig. 2) in good agreement with the 0.5067 slope predicted by the linear approximation of exponential mass-dependent fractionation. Independent measurements of NIST SRM 1486 standards yielded a mean value of: −1.047 ± 0.13‰ 2 SD (n = 101). These values are the same as those measured in the same laboratory at LGLTPE12,24,30,32,33,34,52,53,54,55 and agree well with previously published values from other laboratories31,57,58,59.
Diagenesis
It is well known that diagenetic processes can alter the original isotopic composition of mineralized tissues. That Ca is a major constituent of bioapatite (at a concentration of about 35–40% in weight) represents a solid reason to investigate its isotopic composition in fossil bone and teeth as old as the Cretaceous30,31,53. Here, before chemical purification of Ca isotopes, all samples underwent the standard protocol that is applied for carbon isotopic analyses by removing potential secondary carbonates51. Measuring trace element concentrations requires more than half a milligram—when such elements are present—and therefore necessitates large quantities of sample powder at the cost of precious samples, such as the hominin teeth analyzed in the present study. In this study, our average sample weight uptake was about 100 µg, which is suitable for Ca isotope analysis but obviously not for trace element concentrations. Correlations between trace elements of diagenetic origin and Ca isotopic compositions were assessed in a previous study of fossil mammals from the same Turkana Basin localities29 as those of the hominins analyzed in the present study. We concluded that the impact of diagenesis on our Ca isotopic measurements was minimal29 and by extension, we consider such conclusions applicable to the present hominin dataset.
Comparing East African versus South African datasets
Because a substrate effect cannot be excluded on the fractionation of calcium isotopes, we used associated faunal calcium isotope compositions29,33 to serve as a baseline. This issue has not been explored in detail yet29 but it is worth repeating here that Ca isotopic composition in rocks is rather homogenous60 and a substrate effect may not be pre-eminent in vertebrate tissues. The Turkana fossil assemblage circa 2 Ma includes six felids and four hyaenids calcium isotope values more variable than the range of values of the seven carnivores from South Africa that also includes felids and hyaenids. This dispersion in Ca isotope values is explained by the presence in the Turkana dataset of saber tooth felids, whose diet was specialized as previously discussed29. The fossil browsers from Turkana (–1.16‰, n = 4) versus browsers from South Africa (–1.38‰, n = 7) show different values. Browsers from South Africa consist of one genus only, Tragelaphus, which is known in modern South Africa to include about one-third of C4 component in its diet61 and might explain some of its values to be 44Ca-depleted. The low number of browsers from East Africa that includes two giraffids and two bovids does not permit to satisfyingly compare both browser datasets. Finally, the fossil grazers consist of a larger dataset; taxa from Turkana (–1.25‰, n = 14) versus grazers from South Africa (–1.30‰, n = 10) gave consistent values indicating that beyond some variability that may arise from seasonal variation into food items (although this is restrained by the sampling protocol-see above), nursing processes or other unconstrained environmental processes, it is reasonable to compare both South African and East African palaeoenvironments.
Reporting summary
Further information on research design is available in the Nature Research Reporting Summary linked to this article.
Data availability
The authors declare that all data supporting the findings of this study are included in this published article and its supplementary information files. Source data are provided with this paper. Ca, O and C isotope data of modern and fossil non-primates used in Fig. 2 and Supplementary Figs. 2 and 3 are available from the public repository HAL at https://hal-udl.archives-ouvertes.fr/hal-02568580/. Ca isotope data of fossil taxa from South Africa used in Fig. 3 are available from the open access dataset at https://advances.sciencemag.org/content/5/8/eaax3250/tab-figures-data. Source data are provided with this paper.
References
Cerling, T. E. et al. Stable isotope-based diet reconstructions of Turkana Basin hominins. Proc. Natl. Acad. Sci. USA 110, 10501–10506 (2013).
Wynn, J. G. et al. Geological and palaeontological context of a Pliocene juvenile hominin at Dikika, Ethiopia. Nature 443, 332–336 (2006).
van der Merwe, N., Masao, F. & Bamford, M. Isotopic evidence for contrasting diets of early hominins Homo habilis and Australopithecus boisei of Tanzania. South Afr. J. Sci. 104, 153–155 (2008).
White, T. D. et al. Ardipithecus ramidus and the paleobiology of early hominids. Science 326, 64–86 (2009).
Cerling, T. E. et al. Diet of Paranthropus boisei in the early Pleistocene of East Africa. Proc. Natl. Acad. Sci. USA 108, 9337–9341 (2011).
Henry, A. G. et al. The diet of Australopithecus sediba. Nature 487, 90–93 (2012).
Levin, N., Haile-Selassie, Y., Frost, S. R. & Saylor, B. Z. Dietary change among hominins and cercopithecids in Ethiopia during the early Pliocene. Proc. Natl. Acad. Sci. USA 112, 12304–12309 (2015).
Lee-Thorp, J. et al. Isotopic evidence for an early shift to C4 resources by Pliocene hominins in Chad. Proc. Natl. Acad. Sci. USA 109, 20369–20372 (2012).
Sponheimer, M. et al. Isotopic evidence for dietary variability in the early hominin Paranthropus robustus. Science 314, 980–982 (2006).
Scott, R. S. et al. Dental microwear texture analysis reflects diets of living primates and fossil hominins. Nature 436, 693–695 (2005).
Martin, J. E., Vance, D. & Balter, V. Magnesium stable isotope ecology using mammal tooth enamel. Proc. Natl. Acad. Sci. USA 112, 430–435 (2015).
Martin, J. E., Tacail, T. & Balter, V. Non-traditional isotope perspectives in vertebrate palaeobiology. Palaeontology 60, 485–502 (2017).
Jaouen, K. & Pons, M. L. Potential of non-traditional isotope studies for bioarchaeology. Archaeol. Anthropol. Sci. 9, 1389–1404 (2017).
Tacail, T., Le Houedec, S. & Skulan, J. L. New frontiers in calcium stable isotope geochemistry: perspectives in present and past vertebrate biology. Chem. Geol. https://doi.org/10.1016/j.chemgeo.2020.119471 (2020).
Bourgon, N. et al. Zinc isotopes in Late Pleistocene fossil teeth from a Southeast Asian cave setting preserve paleodietary information. Proc. Natl. Acad. Sci. USA 117, 4675–4681 (2020).
Balter, V. et al. Were Neandertalians essentially carnivores? Sr and Ba preliminary results of the mammalian palaeobiocoenosis of Saint-Césaire. C. R. Acad. Sci. IIA 332, 59–65 (2001).
Sponheimer, M. & Lee-Thorp, J. A. Enamel diagenesis at South African Australopith sites: implications for paleoecological reconstruction with trace elements. Geochim. Cosmochim. Acta 70, 1644–1654 (2006).
Balter, V., Braga, J., Télouk, P. & Thackeray, J. F. Evidence for dietary change but not landscape use in South African early hominins. Nature 489, 558–560 (2012).
Joannes-Boyau, R. et al. Elemental signatures of Australopithecus africanus teeth reveal seasonal dietary stress. Nature 572, 112–115 (2019).
Cerling, T. E. et al. Global vegetation change through the Miocene/Pliocene boundary. Nature 389, 153–158 (1997).
Ungar, P. S., Grine, F. E. & Teaford, M. F. Dental microwear and diet of the Plio-Pleistocene hominin Paranthropus boisei. PLoS ONE 3, e2044 (2008).
Skulan, J. & DePaolo, D. J. Calcium isotope fractionation between soft and mineralized tissues as a monitor of calcium use in vertebrates. Proc. Natl. Acad. Sci. USA 96, 13709–13713 (1999).
Clementz, M. T., Holden, P. & Koch, P. L. Are calcium isotopes a reliable monitor of trophic level in marine settings? Intl. J. Osteoarchaeol. 13, 29–36 (2003).
Martin, J. E., Tacail, T., Adnet, S., Girard, C. & Balter, V. Calcium isotopes reveal the trophic position of extant and fossil elasmobranchs. Chem. Geol. 415, 118–125 (2015).
Reynard, L. M., Henderson, G. M. & Hedges, R. E. M. Calcium isotope ratios in animal and human bone. Geochim. Cosmochim. Acta. 74, 3735–3750 (2010).
Li, Q., Thirwall, M. & Müller, W. Ca isotopic analysis of laser-cut microsamples of (bio)apatite without chemical purification. Chem. Geol. 422, 1–12 (2016).
Melin, A. D. et al. Calcium and carbon stable isotope ratios as paleodietary indicators. Am. J. Phys. Anthropol. 154, 633–643 (2014).
Chu, N. C., Henderson, G. M., Belshaw, N. S. & Hedges, R. E. Establishing the potential of Ca isotopes as proxy for consumption of dairy products. Appl. Geochem. 21, 1656–1667 (2006).
Martin, J. E., Tacail, T., Cerling, T. E. & Balter, V. Calcium isotopes in enamel of modern and Plio-Pleistocene East African mammals. Earth Planet. Sci. Lett. 503, 227–235 (2018).
Hassler, A. et al. Calcium isotopes offer clues on resource partitioning among Cretaceous predatory dinosaurs. Proc. Roy. Soc. B 285, 20180197 (2018).
Heuser, A., Tütken, T., Gussone, N. & Galer, S. J. Calcium isotopes in fossil bones and teeth—Diagenetic versus biogenic origin. Geochim. Cosmochim. Acta. 75, 3419–3433 (2011).
Tacail, T. et al. Assessing human weaning practices with calcium isotopes in tooth enamel. Proc. Natl. Acad. Sci. USA 114, 6268–6273 (2017).
Tacail, T. et al. Calcium isotopic patterns in enamel reflect different nursing behavior among South African early hominins. Sci. Adv. 5, eeax3250 (2019). https://doi.org/10.1126/sciadv.aax3250.
Beynon, A. D., Dean, M. C. & Reid, D. J. Histological study on the chronology of the developing dentition in Gorilla and Orangutan. Am. J. Phys. Anthropol. 86, 189–203 (1991).
Kelley, J. & Schwartz, G. T. Dental development and life history in living African and Asian apes. Proc. Natl. Acad. Sci. 107, 1035–1040 (2010).
Fletcher, A. & Nowell, A. The development of feeding behaviour in wild western lowland gorillas (Gorilla gorilla gorilla). Behaviour 145, 171–193 (2008).
Prado-Martinez, J. et al. Great ape genetic diversity and population history. Nature 499, 471–475 (2014).
Li, Q. et al. Spatially-resolved Ca isotopic and trace element variations in human deciduous teeth record diet and physiological change. Env. Archaeol. In Press, 1–10, https://doi.org/10.1080/14614103.2020.1758988 (2020).
Scott, R. S., Teaford, M. F. & Ungar, P. S. Dental microwear texture and anthropoid diets. Am. J. Phys. Anthropol. 147, 551–579 (2012).
Martin, F. et al. Dietary niches of terrestrial cercopithecines from the Plio-Pleistocene Shungura Formation, Ethiopia: evidence from Dental Microwear Texture Analysis. Sci. Rep. 8, 14052 (2018).
Fashing, P. J., Nguyen, N., Venkataraman, V. V. & Kerby, J. T. Gelada feeding ecology in an intact ecosystem at Guassa, Ethiopia: variability over time and implications for theropith and hominin dietary evolution. Am. J. Phys. Anthropol. 155, 1–16 (2014).
Souron, A. Morphology, diet, and stable carbon isotopes: on the diet of Theropithecus and some limits of uniformitarianism in paleoecology. Am. J. Phys. Anthropol. 166, 261–267 (2018).
Cerling, T. E., Chritz, K. L., Jablonski, N. G., Leakey, M. G. & Manthi, F. K. Diet of Theropithecus from 4 to 1 Ma in Kenya. Proc. Natl. Acad. Sci. USA 110, 10507–10512 (2013).
Grine, F. E., Sponheimer, M., Ungar, P. S., Lee‐Thorp, J. & Teaford, M. F. Dental microwear and stable isotopes inform the paleoecology of extinct hominins. Am. J. Phys. Anthropol. 148, 285–317 (2012).
Leakey, M. G. et al. New fossils from Koobi Fora in northern Kenya confirm taxonomic diversity in early Homo. Nature 488, 201–204 (2012).
Peters, C. R. & Vogel, J. C. Africa’s wild C4 plant foods and possible early hominid diets. J. Hum. Evol. 48, 219–236 (2005).
Schmitt, A.-D. 2016. Earth-Surface Ca Isotopic Fractionations. In Calcium Stable Isotope Geochemistry (eds Tipper, E. T., Schmitt, A. D. and Gussone, N.) 145–172. (Springer, Berlin Heidelberg, 2016).
Yeakel, J. D., Bhat, U., Elliott Smith, E. A. & Newsome, S. Exploring the isotopic niche: isotopic variance, physiological incorporation, and the temporal dynamics of foraging. Front. Ecol. Evol. 4, https://doi.org/10.3389/fevo.2016.00001 (2016).
Wood, B. & Strait, D. Patterns of resource use in early Homo and Paranthropus. J. Hum. Evol. 46, 119–162 (2004).
Wood, B. & Schroer, K. Paranthropus: where do things stand? in Human paleontology and prehistory (eds Marom, A. and Hovers, E.) 95–107 (Springer, Cham, 2017).
Cerling, T. E. et al. Dietary changes of large herbivores in the Turkana Basin, Kenya from 4 to 1 million years ago. Proc. Natl. Acad. Sci. USA 112, 11467–11472 (2015).
Tacail, T., Albalat, E., Télouk, P. & Balter, V. A simplified protocol for measurement of Ca isotopes in biological samples. J. Anal. Atom. Spectrom. 29, 529–535 (2014).
Martin, J. E. et al. Calcium isotopic evidence for vulnerable marine ecosystem structure prior to the K/Pg extinction. Curr. Biol. 27, 1641–1644 (2017).
Balter, V. et al. Calcium stable isotopes place Devonian conodonts as first level consumers. Geochem. Persp. Lett. 10, 36–39 (2019).
Tacail, T., Télouk, P. & Balter, V. Precise analysis of calcium stable isotope variations in biological apatites using laser ablation MC-ICPMS. J. Anal. Atom. Spectrom. 31, 152–162 (2016).
R Core Team. R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. https://www.R-project.org/ (2018).
Heuser, A., Eisenhauer, A., Scholz-Ahrens, K. E. & Schrezenmeir, J. Biological fractionation of stable Ca isotopes in Göttingen minipigs as a physiological model for Ca homeostasis in humans. Isotopes Environ. Health Stud. 52, 633–648 (2016).
Heuser, A. & Eisenhauer, A. The calcium isotope composition (δ44/40Ca) of NIST SRM 915b and NIST SRM 1486. Geostand. Geoanal. Res. 32, 311–315 (2008).
Heuser, A., Schmitt, A., Gussone, N. & Wombacher, F. Analytical Methods. In Calcium Stable Isotope Geochemistry. 23–73 (Springer, Berlin, Heidelberg, 2016).
Tipper, E.T., Schmitt, A. & Gussone, N. Global Ca Cycles: Coupling of Continental and Oceanic Processes. In Calcium Stable Isotope Geochemistry. 173–222 (Springer, Berlin, Heidelberg, 2016). https://doi.org/10.1007/978-3-540-68953-9_6.
Sponheimer, M. et al. Diets of southern African Bovidae: stable isotope evidence. J. Mammal. 84, 471–479 (2003).
Acknowledgements
We thank the National Museums of Kenya and the Kenyan Government for permission to work on this material, and Meave G. Leakey and F. Kyalo Manthi for assistance throughout our studies of hominin diets. We thank Didier Berthet and the Musée des Confluences de Lyon for permission to sample specimens. This work was funded by NSF grant 1740383 and a University of Utah Seed Grant; to T.E.C. and CNRS (Tellus-Rift and INSU INTERRVIE) and ENS-Lyon to J.E.M. and V.B.
Author information
Authors and Affiliations
Contributions
V.B., T.E.C., J.B., T.T., and J.E.M. conceived the study. J.E.M., T.T., T.E.C., and V.B. prepared the samples and measured the isotopic compositions. All co-authors analyzed and discussed the raw data. J.E.M. prepared the draft paper, which was edited by all the co-authors.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Peer review information Nature Communications thanks Shara Bailey, Renaud Joannes-Boyau and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Source data
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Martin, J.E., Tacail, T., Braga, J. et al. Calcium isotopic ecology of Turkana Basin hominins. Nat Commun 11, 3587 (2020). https://doi.org/10.1038/s41467-020-17427-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41467-020-17427-7
- Springer Nature Limited
This article is cited by
-
Combining collagen extraction with mineral Zn isotope analyses from a single sample for robust palaeoecological investigations
Archaeological and Anthropological Sciences (2022)
-
Investigating Isotopic Niche Space: Using rKIN for Stable Isotope Studies in Archaeology
Journal of Archaeological Method and Theory (2022)
-
Contracting eastern African C4 grasslands during the extinction of Paranthropus boisei
Scientific Reports (2021)