Abstract
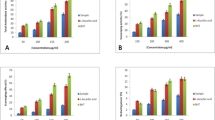
The information available on microalgae-sourced compounds, especially antibiotics and other bioactive compounds, and their potential commercial applications is still insufficient. In this study, antibacterial activity, metabolites, and molecular characterization of Phormidium autumnale, which was isolated from samples collected from different natural freshwater sources in Ankara, Turkey, were investigated. Sequencing results of 16s rDNA confirmed the molecular identification of P. autumnale by 99%. It was determined that the peak values of some phenolic compounds and cyclic peptides were consistent with the 1653–1389 cm−1 band regions in the FTIR spectra of the species. The antibacterial activities of P. autumnale cyanobacteria (CBA) extracts that were obtained by using different solvents were tested on Escherichia coli, Staphylococcus epidermidis, methicillin-resistant (MR) Staphylococcus aureus, Streptococcus agalactiae, and Enterococcus faecalis by using a disc diffusion method. Also, the minimum inhibition concentration (MIC) and antimicrobial indexes of all extracts were determined. It was found that P. autumnale methanol extracts showed antibacterial activity on all test bacteria, whereas acetone extracts showed effects only on E. coli. For the inhibition of MR S. aureus, the control methanol extract was found to give very similar results to those exhibited by the control antibiotics, and the antimicrobial index results were determined to be 58.7–67.5%. According to the results of the analysis of methanol extract, gentisic acid, vanillic acid, 4-hydroxybenzoic acid, p-coumaric acid, and catechin (especially phenolic compounds) were determined to be the active compounds. It can be concluded that P. autumnale is an alternative to current commercial applications as an antibacterial agent in phytotherapy.


Similar content being viewed by others
References
Abedin, R. M. A., & Taha, H. M. (2008). Antibacterial and antifungal activity of cyanobacteria and green microalgae. Evaluation of media components by Plackett- Burman design for antimicrobial activity of Spirulina platensis. Global Journal of Biotechnology & Biochemistry, 3(1), 22–31.
Aliyazıcıoğlu, R., Korkmaz, N., Akkaya, Ş., Şener, Ş. Ö., Özgen, U., & Karaoğlu, Ş. (2018). Investigation of antioxidant, antimicrobial and tyrosinaz inhibitor activities on the aerial parts of Dactilorhiza osmanica. Firat Medical Journal, 23(2), 50–57.
Andersen, R. A., & Kawachi, M. (2005). Traditional microalgae isolation techniques. In R. A. Andersen (Ed.), Algal culturing techniques (pp. 83–100). London: Elsevier Press.
Asadi, A., Khavari-Nejad, R., Soltani, N., Najafi, F., & Molaie-Rad, A. (2011). Physiological and antimicrobial characterizations of some cyanobacteria isolated from the rice fields in Iran. Journal of Agricultural Technology, 7(3), 649–663.
Babić, O., Kovač, D., Rašeta, M., & Šibul, F. (2015). Evaluation of antioxidant activity and phenolic profile of filamentous terrestrial cyanobacterial strains isolated from forest ecosystem. Journal of Applied Phycology, 28, 2333–2342. https://doi.org/10.1007/s10811-015-0773-4.
Bhadury, P., & Wright, P. C. (2004). Exploitation of marine algae: biogenic compounds for potential antifouling applications. Planta, 219, 561–578.
Burja, A. M., Banaigs, B., Abou-Mansour, E., Burgess, J. G., & Wright, P. C. (2001). Marine cyanobacteria–a prolific source of natural products. Tetrahedron, 57, 9347–9377.
Dean, A. P., Martin, M. C., & Sigee, D. C. (2007). Resolution of codominant phytoplankton species in a eutrophic lake using synchrotron-based Fourier transform infrared spectroscopy. Phycologia. https://doi.org/10.2216/06-27.1.
Demiriz, T., Cokmus, C., & Pabuccu, K. (2011). Antimicrobial activity of some algal species belonging to cyanobacteria and chlorophyta. Asian Journal of Chemistry, 23(3), 1384–1386.
Deshmukh, D. V., & Puranik, P. R. (2010). Application of Plackett-Burman design to evaluate media components affecting antibacterial activity of alkaliphilic cyanobacteria isolated from Lonar Lake. Turkish Journal of Biochemistry, 35(2), 114–120.
Duygu, D., Udoh, A. U., Özer Baykal, T., Akbulut, A., Erkaya Açıkgöz, I., Yıldız, K., & Deniz, G. (2012). Fourier transform infrared (FTIR) spectroscopy for identification of Chlorella vulgaris Beijerinck 1890 and Scenedesmus obliquus (Turpin) Kützing 1833. African Journal of Biotechnology, 11(16), 3817–3824.
Eser, F., Yaglioglu, S. A., Aktas, E., Onal, A., Demirtas, I. (2017). Phytochemical content of centaurea polypodiifolia boiss. var. polypodiifolia. International Journal of Secondary Metabolite. https://doi.org/10.21448/ijsm.376888.
Flanga, A., Nigro, E., De Biasi, M. G., et al. (2017). Cyclic peptides as novel therapeutic microbicides: engineering of human defensin mimetics. Molecules. https://doi.org/10.3390/molecules22071217.
Fresnedo, O., Gomez, R., & Serra, J. L. (1991). Carotenoid composition in the cyanobacterium Phormidium laminosum effect of nitrogen stravation. FEBS, 282(2), 300–304.
Ghasemi, Y., Tabatabaei Yazdi, M., Shokravi, S., Soltani, N., & Zarrini, G. (2003). Antifungal and antibacterial activity of paddy-fields cyanobacteria from the north of Iran. Journal of Sciences Islamic Republic of Iran, 14(3), 203–209.
Guillard, R. R. L. (2005). Purification methods for microalgae. In R. A. Andersen (Ed.), Algal culturing techniques (pp. 117–132). London: Elsevier Press.
Guiry, M. D., Guiry, G. M. (2018). AlgaeBase. World-wide electronic publication, National University of Ireland, Galway. http://www.algaebase.org. Accessed 04 November 2018.
Heidari, F., Riahi, H., Yousefzadi, M., & Asadi, M. (2012). Antimicrobial activity of cyanobacteria isolated from hot spring of Geno. Middle-East Journal of Scientific Research, 12(3), 336–339.
Huber-Pestalozzi, G. (1938). Das Phytoplankton Des Süβwassers, 1. Teil E. Schweizerbartsche Verlagsbuchhandlung, Germany.
Huber-Pestalozzi, G. (1955). Das Phytoplankton Des Süβwassers, 4. Teil Euglenophyceen, E. Schweizerbart’sche Verlagsbuchhandlung, Germany.
Huber-Pestalozzi, G. (1982). Das Phytoplankton Des Süβwassers, 8. Teil Conjugatophyceae, Zynematales and Desmidiales, E. Schweizerbart’sche Verlagsbuchhandlung, Germany.
Hur, S. B., Bae, J. H., Youn, J. Y., Jo, M. J. (2015). KMMCC-Korea marine microalgae culture center: list of strains, 2nd edition. Algae, S1-S188.
Jaki, B., Heilmann, J., Linden, A., Volger, B., & Sticher, O. (2000). Novel extra cellular diterpenoids with biological activity from the cyanobacterium Nostoc commune. Journal of Natural Products, 63, 339–343.
Jerez-Martel, I., Garcia-Poza, S., Rodriguez-Martel, G., Rico, M., Afonso-Olivares, C., & Gómez-Pinchetti, J. L. (2017). Phenolic profile and antioxidant activity of crude extracts from microalgae and cyanobacteria strains. Hindawi Journal of Food Quality. https://doi.org/10.1155/2017/2924508.
Malathi, T., Ramesh Babu, M., Mounika, T., Snehalatha, D., & Digamber Rao, B. (2014). Screening of cyanobacterial strains for antibacterial activity. Phykos, 44(2), 6–11.
Martins, J., Peixe, L., & Vasconcelos, V. M. (2011). Unraveling cyanobacteria ecology in wastewater treatment plants (WWTP). Microbial Ecology, 62, 241–256.
Meijer, N. (2017). The relationship between enhanced Phormidium growth and fine sediment depositon in New Zealand Rivers. Desertion, University College of Southeast Norway Faculty of Arts and Science.
Mundt, S., Kreitlow, S., Nowotny, A., & Effmert, U. (2001). Biological and pharmacological investigation of selected cyanobacteria. International Journal of Hygiene and Environmental Health, 203, 327–334.
Namikoshi, M., & Rinehart, K. L. (1996). Bioactive compounds produced by Cyanobacteria. Journal of Industrial Microbiology, 17, 373–384.
Nauman, D. (2002). Infrared spectroscopy in microbiology. In R. A. Meyers (Ed.), Encyclopedia of analytical chemistry (pp. 102–131). Chichester: John Wiley & Sons, Ltd..
Pabuçcu, K., & Demiriz Yücer, T. (2018). Investigation of the medical features of Phormidium species. Gaziosmanpasa Journal of Scientific Research, 7(1), 101–115.
Pandey, V. D. (2015). Cyanobacterial natural products as antimicrobial agents. International Journal of Current Microbiology and Applied Sciences, 4(1), 310–317.
Panjiar, N., Mishra, S., Yadav, A. N., & Verma, P. (2018). Functional foods from cyanobacteria: an emerging source for functional food products of pharmaceutical importance. In V. K. Gupta, H. Treichel, V. Shapaval, L. A. Oliveira, & M. G. Tuohy (Eds.), Microbial functional foods and nutraceuticals (First ed.). USA: John Wiley & Sons. https://doi.org/10.1002/9781119048961.ch2.
Parsons, T. R., & Strickland, J. D. H. (1963). Discussion of spectrophotometric determination of marine plant pigments, with revised equations for ascertaining chlorophylls and carotenoids. Journal of Marine Research, 21(3), 115–163.
Ponnuswamy, I., Madhavan, S., & Shabudeen, S. (2013). Isolation and characterization of green microalgae for carbon sequestration, waste water treatment and bio-fuel production. International Journal of Bio-Science and Bio-Technology, 5(2), 17–26.
Poon, C. K., Chapman, R., Jolliffe, K. A., & Perrie, S. (2012). Pushing the limits of copper mediated azide–alkyne cycloaddition (CuAAC) to conjugate polymeric chains to cyclic peptides. Polymer Chemistry, 3, 1820–1826.
Prakash, N. K., Bhuvaneswari, S., et al. (2012). A study on antibacterial activitiy of common weeds in northern districts of Tamil Nadu, India. Research Journal of Medical Plant. https://doi.org/10.3923/rjmp.2012.341.345.
Rajeev, K. J., & Xu, Z. (2004). Biomedical compounds from marine organisms. Marine Drugs, 2, 123–146.
Rania, M. A., & Taha, A. H. M. (2008). Antibacterial and antifungal activity of Cyanobacteria and green microalgae, evaluation of medium components by Placket-Burman design for antimicrobial activity of Spirulina platensis. Global Journal of Biotechnology & Biochemistry, 3(1), 22–31.
Rodriguez-Meizoso, I., Jaime, L., Santoyo, S., Cifuentes, A., Garcia-Blairsy Reina, G., Senorans, F. J., & Ibanez, E. (2008). Pressurized fluid extraction of bioactive compounds from Phormidium species. Journal of Agricultural and Food Chemistry, 56, 3517–3523.
Santhose, I., Gnanadoss, J., Ramganesh, S., & Elumalai, S. (2011). Enhanced carotenoid synthesis of Phormidium sp. in stressed conditions. Journal of Experimental Science, 2(3), 38–44.
Sasidharan, S., Darah, I., & Noordin, M. K. (2010). In vitro antimicrobial activity against Pseudomonas aeruginosa and acute oral toxicity of marine algae Gracilaria changii. New Biotechnology. https://doi.org/10.1016/j.nbt.2010.02.002.
Schlegel, I., Doan, N. T., de Chazal, N., & Smith, G. D. (1999). Antibiotic activity of new cyanobacterial isolates from Australia and Asia against green algae and cyanobacteria. Journal of Applied Phycology, 10(5), 471–479.
Shamchi, M. P. (2016). Investigation of cyanobacteria and some algal species bioactive compounds. Desertion, Hacettepe University.
Sigee, D. C., Dean, A., Levado, E., & Tobin, M. J. (2002). Fourier-transform infrared microscopy of Pediastrum dublex: characterization of a micro-population isolated from a eutrophic lake. European Journal of Phycology, 37, 19–26.
Signh, Y. P., Das, R., & Singh, R. A. (2007). Numerical simulation of the internal vibrations of COOH group in amino-salicylic acids. African Journal of Biochemistry Research, 1(2), 19–23.
Sundaramanickam, A., Palanivel, S., Shekhar, S., Kumaresan, S., & Balasubramanian, T. (2015). In vitro evaluation of antimicrobial activity of some selected cyanobacterial extracts against human pathogens. IJAPBC, 4(1), 36–43.
Sundararaman, M., & Sekar, S. (2001). Biotechnological potentials of cyanobacteria. Jaipur: Algal Biotechnology, Pointer Publishers.
Swisłocka, R., Kowczyk-Sadowy, M., & Lewandowski, W. (2012). Spectroscopic (FT-IR, FT-Raman, 1H and 13C NMR) and theoretical studies of p-coumaric acid and alkali metal p-coumarates. Spectroscopy. https://doi.org/10.3233/SPE-2012-0568.
Thajuddin, N., & Subramanian, G. (2005). Cyanobacterial biodiversity and potential applications in biotechnology. Current Science, 89(1), 47–57.
Tiwari, A., & Sharma, D. (2013). Antibacterial activity of bloom farming Cyanobacteria against clinically isolated human pathogenic microbes. Journal of Algal Biomass Utilization., 4(1), 83–89.
Trivedi, M. K., Branton, A., Trivedi, D., Shettigar, H., Bairwa, K., & Jana, S. (2015). Fourier transform infrared and ultraviolet-visible spectroscopic characterization of biofield treated salicylic acid and sparfloxacin. Natural Products Chemistry & Research. https://doi.org/10.4172/2329-6836.1000186.
Acknowledgments
We would like to thank Prof. Dr. İbrahim Demirtaş from Çankırı Karatekin University for the HPLC analysis and Andrew P. Dean from the University of Manchester for the PCR analysis.
Funding
This study was supported by the Gazi University Scientific Projects Research Management Funds (No. 04-2007-28).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interests
The authors declare that they have no conflict of interest.
Ethical approval
This article does not contain any studies with animals.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Highlights
• There is insufficient knowledge about the bioactive compounds of Cyanobacteria.
• Cyanobacteria have been used in many commercial fields, especially in phytotherapy.
• Many antimicrobial compounds have lost some of their efficacy due to various reasons.
• Cyanobacteria have become important subjects of alternative biodegradable and broad spectrum for researchers.
• Phormidium autumnale is an alternative to current commercial applications as an antibacterial agent in phytotherapy.
Rights and permissions
About this article
Cite this article
Yalcin, D., Türk Katircioğlu, H., Özer, T. et al. Evaluation of phytotherapeutic activities and phytochemical content of Phormidium autumnale Gomont from natural freshwater sources. Environ Monit Assess 192, 244 (2020). https://doi.org/10.1007/s10661-020-8207-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10661-020-8207-4