Abstract
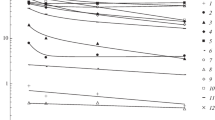
A combined approach consisting of monitoring and thermodynamic modeling was used in order to calculate the concentration of trace element species in water samples of a broad salinity range and to explain their chemical behaviour. The study was performed on water samples (fresh, marine, hyper-saline) taken from the area of Burgas Bay, Bulgaria. The ion association model based on Debye–Hückel theory using the sst2008.dat database and the ion interaction model based on Pitzer theory using a new pit2010.dat database were compared and combined for the purposes of this study. The new pit2010.dat database combines the sst2008.dat database and the pitzer.dat database of the PHREEQCI computer program as well as the thermodynamic data for the elements Fe, Mn, Cu, Zn, Cd and Pb and their Pitzer ion interaction parameters. The results showed that: (1) the predominant species in fresh waters were free ions of Mn2+ (73.6%), Zn2+ (58.0%) and Cd2+ ions (78.3%) as well as carbonate species CuCO\(_{3}^{0}\) (81.8%), PbCO\(_{3}^{0}\) (77.2%) and hydroxy species Fe(OH)\(_{3}^{0}\) (55.2%) and Fe(OH)\(_{2}^{+}\)(35.6%); (2) an increase in chloride species MeCl\(_{n}^{2-n }\)(n = 1–4, Me = Mn, Zn, Cu, Pb and Cd) and of the hydroxy species Fe(OH)\(_{2}^{+ }\) for Fe was calculated for sea and hyper-saline water.
Similar content being viewed by others
References
Allison, J. A., Brown, D. S., & Novo-Gradac, K. J. (1991). MINTEQA2/PRODEFA2, A geochemical assessment model for environmental systems: Version 3.0 user’s manual (pp. 106). U.S. Environ. Prot. Agency, EPA/600/3-91/021.
ATSDR (2000). Draft toxicological profile for manganese. Atlanta USA: US Department of Health and Human Environ Monit Assess Services, Public Health Service, Retrieved from http://www.atsdr.cdc.gov/toxprofiles/tp151.html.
Ball, J. W., & Nordstrom, D. K. (1991). WATEQ4F-User’s manual (pp. 185). U.S., Geological Survey Open-File Report 90–129.
Brimblecombe, P., & Clegg, S. L. (1990). The solubility and behavior of acid gases in the marine aerosol. Journal of Atmospheric Chemistry, 7, 1.
Christov, Chr. (2004). Pitzer ion-interaction parameters for Fe(II) and Fe(III) in the quinary Na + K + Mg + Cl + SO4 + H2O system at T = 298.15 K. Journal of Chemical Thermodynamics, 36, 223–235.
Clegg, S. L., & Brimblecombe, P. (1990). The solubility of volatile electrolytes in multicomponent solutions with atmospheric applications. In: D. C. Melchior, & R. L. Bassett (Eds.), Chemical modeling in aqueous systems II. Washington D. C. : ACS.
Einav, R., & Lokiec, F. (2003). Environmental aspects of a desalination plant in Ashkelon. Desalination, 156, 79–85.
Gilberto, C., Maddux, W. S. , & Burkholder, P. R. (1970). Some consequences of brine pollution in the Bahía Fosforescente, PuertoRico. Limnology and Oceanography, 1970, 15(2), 246–249.
Goldberg, R. N. (1979). Evaluated activity and osmotic coefficients for aqueous solutions: bi-univalent compounds of lead, copper, manganese, and uranium. Journal of Physical and Chemical Reference Data, 8, 1005.
Goldberg, R. N. (1981). Evaluated activity and osmotic coefficients for aqueous solutions: Bi-univalent compounds of zinc, cadmium, and ethylene bis(trimethylammonium) chloride and iodide. Journal of Physical and Chemical Reference Data, 10, 1.
Grasshoff, K., Kremling, K., & Ehrhardt, M. (1999). Methods in seawater analysis. Germany: Wiley.
Grenthe, I., & Puigdomenech, I. (1997). Modelling in aquatic chemistry (pp. 724). OECD Publications.
Hamer, W. J., & Wu, Y. C. (1972). Osmotic Coefficients and Mean Activity Coefficients of Uni-univalent Electrolytes in Water at 25°C. Journal of Physical and Chemical Reference Data, 1, 1047.
Harvie, C. E., & Weare, J. H. (1980). The prediction of mineral solubilities in natural waters: The Na-K-Mg-Ca-Cl-SO4-H2O system from zero to high concentration at 25°C. Geochimica et Cosmochimica Acta, 44, 981–997.
Harvie, C. E., Eugster, H. P., & Weare, J. H. (1982). Mineral equilibria in the six component seawater systems Na-K-Mg-Ca-Cl-SO4-H2O at 25°C. Geochimica et Cosmochimica Acta, 46, 1603.
Harvie, C. E., Moller, N., & Weare, J. H. (1984). The prediction of mineral solubilities in natural waters: The Na-K-Mg-Ca-H-Cl-SO4 -OH-HCO3 -CO3 -CO2 -H2O system to high ionic strengths at 25°C. Geochimica et Cosmochimica Acta, 48, 723–751.
Klopman, G. (1968). Chemical reactivity and the concept of charge- and frontier-controlled reactions. Journal of the American Chemical Society, 90, 223–234.
Leonova, G. A., Bobrov, G. A., Bogush, A. A., Bychinskii, V. A., & Anoshin, G. N. (2007). Geochemical characteristics of the Modern State of Salt Lakes in Altai Krai. Geochemistry International, 45(10), 1025–1039.
Meerganz von Medeazza, G. L. (2005). “Direct” and socially-induced environmental impacts of desalination. Desalination, 185, 57–70.
Millero, F. J. (2001). Speciation of metals in natural waters. Geochemical Transactions, 2, 88.
Millero, F. J., & Hawke, D. J. (1992). Ionic interactions of divalent metals in natural waters. Marine Chemistry, 40, 19–48.
Millero, F. J., & Pierrot, D. (1998). A chemical equilibrium model for natural waters. Aquatic Geochemistry, 4, 153–199.
Parkhurst, D. L. (1995). User’s guide to PHREEQC-A computer program for speciation, reaction-path, advective-transport, and inverse geochemical calculations (pp. 143). U.S. Geological Survey Water-Resources Investigations Report 95-4227.
Pearson, R. (1973). Hard and soft acids and bases. Stroudsburg: Hutchinson.
Pitzer, K. S. (1972). Thermodynamic properties of aqueous solutions of bivalent sulphates. Journal of the Chemical Society. Faraday Transactions, 2(68), 101.
Pitzer, K. S. (1979). Theory–ion interaction approach. In: R. M. Pytkowicz (Ed.), Activity coefficients in electrolyte solutions (Vol. 1, pp. 157–208). Boca Raton: CRC.
Pitzer, K. S. (1991). Activity coefficients in electrolyte solutions (2nd ed.). Boca Raton: CRC.
Pitzer, K. S., & Mayorga, G. (1973). Thermodynamics of electrolytes. II. activity and osmotic coefficients for strong electrolytes with one or both ions univalent. Journal of Physical Chemistry, 77, 2300–2308.
Plummer, L. N., Parkhurst, D. L., Fleming, G. W., & Dunkle, S. A. (1988). A computer program incorporating Pitzer’s equations for calculation of geochemical reactions in brines (pp. 310). U.S. Geological Survey Water-Resources Investigations Report 88-4153.
Purnama, A., Al-Barwani, H. H., & Al-Lawatia, M. (2003). Modeling dispersion of brine waste discharges from a coastal desalination plant. Desalination, 155, 41–47.
Rabadjieva, D., Tepavitcharova, S., Todorov, T., Dassenakis, M., Paraskevopoulou, V., & Petrov, M. (2009). Thermodynamic modeling of inorganic chemical speciation in river waters affected by mine water discharges. Euro-Asian Journal of Sustainable Energy Development Policy, 3, 15–25.
Reed, D. T., Clark, S. B., & Linfeng, R. (Eds.) (1999). Actinide speciation in high ionic strength media: Experimental and modeling approaches to predicting actinide speciation and migration in the subsurface (pp. 278). Dordrecht: Kluwer.
Riley, J. P., & Taylor, D. (1986). Chelating resins for the concentration of trace elements from sea water and their analytical use in conjunction with AAS. Analytica Chimica Acta, 40, 479–484.
Robinson, R. A., & Stokes, R. H. (1959). Electrolyte solutions (2nd ed.). London: Butterworths.
Shumilin, E. N., Mironenko, M. V., Ryzhenko, B. M., & Grajeda Munoz, M. (2003). Speciation of trace elements in brines from the concentration ponds of Exportadora del Sal, Guerrero Negro, Baja California Sur, Mexico. Geochemistry International, 41(3), 295–304.
Simkiss, K., & Taylor, M. G. (1995). Transport of metals across membranes. In A. Tessier, & D. R. Turner (Eds.), Metal speciation and bioavailability in aquatic systems. IUPAC Series on Analytical and Physical Chemistry of Environmental Systems (Vol. 3). Hoboken: Wiley.
Sposito, G., & Traina, S. J. (1987). Ion-association model for highly saline, sodium chloride-dominated waters. Journal of Environmental Quality, 16(1), 80–85.
Tepavitcharova, S., Balarew, Chr., Rull, F., Rabadjieva, D., & Iliev, A. (2005). Raman spectroscopic studies of ion association in the Na+, Mg2+/Cl−, SO\(_{4}^{2-}\)/H2O system. Journal of Raman Spectroscopy, 36(9), 891–897.
Wu, Y. C., & Hamer, W. J. (1980). Revised values of the osmotic coefficients and mean activity coefficients of sodium nitrate in water at 25°C. Journal of Physical and Chemical Reference Data, 9, 513.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Tepavitcharova, S., Todorov, T., Rabadjieva, D. et al. Chemical speciation in natural and brine sea waters. Environ Monit Assess 180, 217–227 (2011). https://doi.org/10.1007/s10661-010-1783-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10661-010-1783-y