Summary
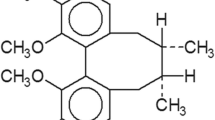
Gliomas account for nearly 70% of the central nervous system tumors and present a median survival of approximately 12–17 months. Studies have shown that administration of novel natural antineoplastic agents is been highly effective for treating gliomas. This study was conducted to investigate the antitumor potential (in vitro and in vivo) of Miconia chamissois Naudin for treating glioblastomas. We investigated the cytotoxicity of the chloroform partition and its sub-fraction in glioblastoma cell lines (GAMG and U251MG) and one normal cell line of astrocytes. The fraction showed cytotoxicity and was selective for tumor cells. Characterization of this fraction revealed a single compound, Matteucinol, which was first identified in the species M. chamissois. Matteucinol promoted cell death via intrinsic apoptosis in the adult glioblastoma lines. In addition, Matteucinol significantly reduced the migration, invasion, and clonogenicity of the tumor cells. Notably, it also reduced tumor growth and angiogenesis in vivo. Moreover, this agent showed synergistic effects with temozolomide, a chemotherapeutic agent commonly used in clinical practice. Our study demonstrates that Matteucinol from M chamissois is a promising compound for the treatment of glioblastomas and may be used along with the existing chemotherapeutic agents for more effective treatment.






Similar content being viewed by others
References
Schneider T, Mawrin C, Scherlach C, Skalej M, Firsching R (2010) Gliomas in adults. Dtsch Arztebl Int 107(45):799–807
Hour M-J, Liu W-T, Lu I-C, Kuo S-C, Gean P-W (2014) Aggravated DNA damage as a basis for enhanced glioma cell killing by MJ-66 in combination with minocycline. Am J Cancer Res 4(5):474–483
Sitarek P, Skała E, Toma M, Wielanek M, Szemraj J, Skorski T, Białas AJ, Sakowicz T, Kowalczyk T, Radek M (2016) Transformed root extract of Leonurus sibiricus induces apoptosis through intrinsic and extrinsic pathways in various grades of human glioma cells. Pathol Oncol Res 23(3):679–687
Zhang Y, Xie R-F, Xiao Q-G, Li R, Shen X-L, Zhu X-G (2014) Hedyotis diffusa Willd extract inhibits the growth of human glioblastoma cells by inducing mitochondrial apoptosis via AKT/ERK pathways. J Ethnopharmacol 158:404–411
de Rezende AR, Romero R, Goldenberg R (2014) Sinopse de Miconia seção Miconia Dc. (Melastomataceae) no estado de Minas Gerais, Brasil Synopsis of Miconia section Miconia Dc. (Melastomataceae) in the state of Minas Gerais, Brazil. Biosci J 30(1)
Chowdhury SA, Kishino K, Satoh R, Hashimoto K, Kikuchi H, Nishikawa H, ShiratakI Y, Sakagami H (2005) Tumor-specificity and apoptosis-inducing activity of stilbenes and flavonoids. Anticancer Res 25(3B):2055–2063
Kuster R, Caxito M, Sabino K, da Costa H, Tose L, Romão W, Vaz B, Silva A (2015) Identification of maloyl glucans from Euphorbia tirucalli by ESI(−)-FT-ICR MS analyses. Phytochem Lett 12:209–214
Kill JB, Oliveira IF, Tose LV, Costa HB, Kuster RM, Machado LF, Correia RM, Rodrigues RR, Vasconcellos GA, Vaz BG (2016) Chemical characterization of synthetic cannabinoids by electrospray ionization FT-ICR mass spectrometry. Forensic Sci Int 266:474–487
Amitani M, Cheng K-C, Asakawa A, Amitani H, Kairupan TS, Sameshima N, Shimizu T, Hashiguchi T, Inui A (2015) Allantoin ameliorates chemically-induced pancreatic β-cell damage through activation of the imidazoline I3 receptors. PeerJ. 3:e1105
Silva-Oliveira RJ, Lopes GF, Camargos LF, Ribeiro AM, Santos FV, Severino RP, VGP S, Terezan AP, Thomé RG, Santos HB (2016) Tapirira guianensis Aubl. Extracts inhibit proliferation and migration of oral cancer cells lines. Int J Mol Sci 17(11):1839
Chuang YF, Huang SW, Hsu YF, Yu MC, Ou G, Huang WJ, Hsu MJ (2017) WMJ-8-B, a novel hydroxamate derivative, induces MDA-MB-231 breast cancer cell death via SHP-1-STAT3-survivin cascade. Br J Pharmacol 174(17):2941–2961
Li Y, Guo G, Song J, Cai Z, Yang J, Chen Z, Wang Y, Huang Y, Gao Q (2017) B7-H3 promotes the migration and invasion of human bladder cancer cells via the PI3K/Akt/STAT3 signaling pathway. J Cancer 8(5):816–824
Chou T-C, Talalay P (1984) Quantitative analysis of dose-effect relationships: the combined effects of multiple drugs or enzyme inhibitors. Adv Enzym Regul 22:27–55
Chou T-C, Talaly P (1977) A simple generalized equation for the analysis of multiple inhibitions of Michaelis-Menten kinetic systems. J Biol Chem 252(18):6438–6442
Hagedorn M, Javerzat S, Gilges D, Meyre A, de Lafarge B, Eichmann A, Bikfalvi A (2005) Accessing key steps of human tumor progression in vivo by using an avian embryo model. Proc Natl Acad Sci U S A 102(5):1643–1648
Zhao J, Ding HX, Zhao DG, Wang CM, Gao K (2012) Isolation, modification and cytotoxic evaluation of flavonoids from Rhododendron hainanense. J Pharm Pharmacol 64(12):1785–1792
Van T et al (2018) Isolation and purification of potent growth inhibitors from Piper methysticum root. Molecules 23(8):1907
Perrin SL, Samuel MS, Koszyca B, Brown MP, Ebert LM, Oksdath M, Gomez GA (2019) Glioblastoma heterogeneity and the tumour microenvironment: implications for preclinical research and development of new treatments. Biochemical Society Transactions. BST20180444
Park C-K, Lee SH, Kim TM, Choi SH, Park SH, Heo DS, Kim IH, Jung HW (2013) The value of temozolomide in combination with radiotherapy during standard treatment for newly diagnosed glioblastoma. J Neuro-Oncol 112(2):277–283
Zhang J, Stevens FG, Malcolm, Bradshaw D, Tracey (2012) Temozolomide: mechanisms of action, repair and resistance. Curr Mol Pharmacol 5(1):102–114
Johannessen TCA, Bjerkvig R (2012) Molecular mechanisms of temozolomide resistance in glioblastoma multiforme. Expert Rev Anticancer Ther 12(5):635–642
Zhu Y, Fang J, Wang H, Fei M, Tang T, Liu K, Zhou Y (2018) Baicalin suppresses proliferation, migration, and invasion in human glioblastoma cells via Ca2+-dependent pathway. Drug Des Dev Ther 12:3247
Allan LA, Morrice N, Brady S, Magee G, Pathak S, Clarke PR (2003) Inhibition of caspase-9 through phosphorylation at Thr 125 by ERK MAPK. Nat Cell Biol 5(7):647–654
Zhang D et al (2013) Two new C-methyl flavanones from the rhizomes and frond bases of Matteuccia struthiopteris. J Asian Nat Prod Res 15(11):1163–1167
Hausenloy DJ, Yellon DM (2004) New directions for protecting the heart against ischaemia–reperfusion injury: targeting the reperfusion injury salvage kinase (RISK)-pathway. Cardiovasc Res 61(3):448–460
Steelman LS, Chappell WH, Abrams SL, Kempf CR, Long J, Laidler P, Mijatovic S, Maksimovic-Ivanic D, Stivala F, Mazzarino MC (2011) Roles of the Raf/MEK/ERK and PI3K/PTEN/Akt/mTOR pathways in controlling growth and sensitivity to therapy-implications for cancer and aging. Aging (Albany NY) 3(3):192
Fernandez-Capetillo O, Chen HT, Celeste A, Ward I, Romanienko PJ, Morales JC, Naka K, Xia Z, Camerini-Otero RD, Motoyama N, Carpenter PB, Bonner WM, Chen J, Nussenzweig A (2002, 2002) DNA damage-induced G2–M checkpoint activation by histone H2AX and 53BP1. Nat Cell Biol 4(12):993–997
Ivashkevich A, Redon CE, Nakamura AJ, Martin RF, Martin OA (2012) Use of the γ-H2AX assay to monitor DNA damage and repair in translational cancer research. Cancer Lett 327(1):123–133
Chang Y-L et al (2017) The synergistic effects of valproic acid and fluvastatin on apoptosis induction in glioblastoma multiforme cell lines. Int J Biochem Cell Biol 92:155–163
Yan X et al (2014) Ginsenoside rb1 protects neonatal rat cardiomyocytes from hypoxia/ischemia induced apoptosis and inhibits activation of the mitochondrial apoptotic pathway. Evid Based Complement Alternat Med
Gao G, Jiang YW, Yang J, Wu FG (2017) Mitochondria-targetable carbon quantum dots for differentiating cancerous cells from normal cells. Nanoscale. 9(46):18368–18378
Hanahan D, Weinberg RA (2011) Hallmarks of cancer: the next generation. Cell. 144(5):646–674
Huse JT, Holland E, Deangelis LM (2013) Glioblastoma: molecular analysis and clinical implications. Annu Rev Med 64:59–70
Franken NA, Rodermond HM, Stap J, Haveman J, Van Bree C (2006) Clonogenic assay of cells in vitro. Nat Protoc 1(5):2315–2319
Teicher BA (2009) In vivo/ex vivo and in situ assays used in cancer research: a brief review. Toxicol Pathol 37(1):114–122
Hsiao YC, Hsieh YS, Kuo WH, Chiou HL, Yang SF, Chiang WL, Chu SC (2007) The tumor-growth inhibitory activity of flavanone and 2′-OH flavanone in vitro and in vivo through induction of cell cycle arrest and suppression of cyclins and CDKs. J Biomed Sci 14(1):107–119
Kargiotis O, Rao J, Kyritsis A (2006) Mechanisms of angiogenesis in gliomas. J Neuro-Oncol 78(3):281–293
Onishi M, Ichikawa T, Kurozumi K, Date I (2011) Angiogenesis and invasion in glioma. Brain Tumor Pathol 28:13–24
Grogan PT, Sarkaria JN, Timmermann BN, Cohen MS (2014) Oxidative cytotoxic agent withaferin A resensitizes temozolomide-resistant glioblastomas via MGMT depletion and induces apoptosis through Akt/mTOR pathway inhibitory modulation. Investig New Drugs 32(4):604–617
Lee HS, Cho HJ, Yu R, Lee KW, Chun HS, Park JHY (2014) Mechanisms underlying apoptosis-inducing effects of Kaempferol in HT-29 human colon cancer cells. Int J Mol Sci 15(2):2722–2737
Funding
The work was supported by Minas Gerais State Research Foundation (FAPEMIG), National Council for Scientific and Technological Development (CNPq),and supported partially by grantsfrom the FINEP (MCTI/ FINEP/MS/SCTIE/DECIT-01/2013 - FPXII-BIOPLAT). This work was conducted during a scholarship supported by the International Cooperation Program CAPES (Brazilian Federal Agency for Support and Evaluation of Graduate Education within the Ministry of Education of Brazil) at the Federal University of São João del Rei.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
Author Silva, A.G. declares that she has no conflict of interest. Author Saito, V.A.O.S. declares that she has no conflict of interest. Author Oliveira R.J.S. declares that he has no conflict of interest. Author Rezende, A.R. declares that he has no conflict of interest. Author Chagas R.C.R. declares that he has no conflict of interest. Author Pimenta, L.P.S. declares that she has no conflict of interest. Author Romão, W. declares that he has no conflict of interest. Author Santos, H.B. declares that he has no conflict of interest. Author Thomé R.G. declares that he has no conflict of interest. Author Reis, R. declares that he has no conflict of interest. Author Ribeiro, R.I.M.A. declares that she has no conflict of interest.
Ethical approval
This article has a study with animals, ethical approval was obtained by the Ethics Committee of the Foundation Pio XII IRCAD Brazil under protocol number 027/2016. All applicable international, national, institutional guidelines for the care and use of animals were followed.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Table S1
(PDF 249 kb)
Rights and permissions
About this article
Cite this article
Silva, A.G., Silva, V.A.O., Oliveira, R.J.S. et al. Matteucinol, isolated from Miconia chamissois, induces apoptosis in human glioblastoma lines via the intrinsic pathway and inhibits angiogenesis and tumor growth in vivo. Invest New Drugs 38, 1044–1055 (2020). https://doi.org/10.1007/s10637-019-00878-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10637-019-00878-1