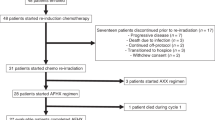
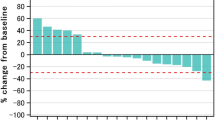
Summary
Purpose Vorinostat is a potent HDAC inhibitor that sensitizes head and neck squamous cell carcinoma (HNSCC) to cytotoxic therapy while sparing normal epithelium. The primary objective of this Phase I study was to determine the maximally tolerated dose (MTD) and safety of Vorinostat in combination with standard chemoradiation therapy treatment in HNSCC. Patients and Methods Eligible patients had pathologically confirmed Stage III, IVa, IVb HNSCC, that was unresectable or borderline resectable involving the larynx, hypopharynx, nasopharynx, and oropharynx. Vorinostat was administered at the assigned dosage level (100-400 mg, three times weekly) in a standard 3 + 3 dose escalation design. Vorinostat therapy began 1 week prior to initiation of standard, concurrent chemoradiation therapy and continued during the entire course of therapy. Results Twenty six patients met eligibility criteria and completed the entire protocol. The primary tumor sites included tonsil (12), base of tongue (9), posterior pharyngeal wall (1), larynx (4) and hypopharynx (3). Of the 26 patients, 17 were HPV-positive and 9 were HPV-negative. The MTD of Vorinostat was 300 mg administered every other day. Anemia (n = 23/26) and leukopenia (n = 20/26) were the most commonly identified toxicities. The most common Grade3/4 events included leukopenia (n = 11) and lymphopenia (n = 17). No patient had Grade IV mucositis, dermatitis or xerostomia. The median follow time was 33.8 months (range 1.6–82.9 months). Twenty four of 26 (96.2%) patients had a complete response to therapy. Conclusion Vorinostat in combination with concurrent chemoradiation therapy is a safe and highly effective treatment regimen in HNSCC. There was a high rate of complete response to therapy with toxicity rates comparable, if not favorable to existing therapies. Further investigation in Phase II and III trials is strongly recommended.






Similar content being viewed by others
References
Leemans CR, Braakhuis BJM, Brakenhoff RH (2011) The molecular biology of head and neck cancer. Nat Rev Cancer 11(1):9–22
Mehanna H, Paleri V, West CML, Nutting C (2010) Head and neck cancer-part 1: epidemiology, presentation, and prevention. BMJ 341:c4684
Miller KD, Siegel RL, Lin CC, Mariotto AB, Kramer JL, Rowland JH, Stein KD, Alteri R, Jemal A (2016) Cancer treatment and survivorship statistics. Cancer J Clin 66:271–289
Chaturvedi AK, Graubard BI, Broutian T, Pickard RKL, Tong ZY, Xiao W, Kahle L, Gillison ML (2015) NHANES 2009-2012 findings: association of sexual behaviors with higher prevalence of oral oncogenic human papillomavirus infections in U.S. men. Cancer Res 75:2468–2477
Kalavrezos N, Bhandari R (2010) Current trends and future perspectives in the surgical management of oral cancer. Oral Oncol 46:429–432
Edwards BK, Brown ML, Wingo PA et al (2005) Annual report to the nation on the status of cancer, 1975–2002, featuring population based trends in cancer treatment. J Natl Cancer Inst. Oct 5 97(19):1407–1427
Ang KK, Harris J, Wheeler R, Weber R, Rosenthal DI, Nguyen-Tân PF, Westra WH, Chung CH, Jordan RC, Lu C, Kim H, Axelrod R, Silverman CC, Redmond KP, Gillison ML (2010) Human papillomavirus and survival of patients with oropharyngeal cancer. N Engl J Med 363:24–35
Carvalho AL, Hishimoto IN, Califano JA et al (2005) Trends in incidence and prognosis for head and neck cancer in the United States: a site-specific analysis of the SEER database. Int J Cancer 114:806–881
Preuss SF, Quante G, Semrau R, Mueller RP, Klussmann JP, Guntinas-Lichius O (2007) An analysis of surgical complications, morbidity, and cost calculation in patients undergoing multimodal treatment for operable oropharyngeal carcinoma. Laryngoscope 117(1):101–105
Sundaram K, Schwartz J, Har-El G et al (2005) Carcinoma of the oropharynx: factors affecting outcome. Laryngoscope 115(9):1536–1542
Wolf GT, Fisher SG, Hong WK et al (1991) Induction chemotherapy plus radiation compared with surgery plus radiation in patients with advanced laryngeal cancer: the department of veterans affairs laryngeal cancer study group. N Engl J Med 324:1685–1690
Urba S, Wolf G, Eisbruch A, Worden F, Lee J, Bradford C, Teknos T, Chepeha D, Prince M, Hogikyan N, Taylor J (2006) Single-cycle induction chemotherapy selects patients with advanced laryngeal cancer for combined chemoradiation: a new treatment paradigm. J Clin Oncol 24:593–598
Worden FP, Kumar B, Lee JS et al (2008) Chemoselection as a strategy for organ preservation in advanced oropharynx cancer: response and survival positively associated with HPV16 copy number. J Clin Oncol 26(19):3138–3146
Adelstein DJ, Saxton JP, Lavertu P, Rybicki LA, Esclamado RM, Wood BG, Strome M, Carroll MA (2002) Maximizing local control and organ preservation in stage IV squamous cell head and neck cancer with hyperfractionated radiation and concurrent chemotherapy. J Clin Oncol 20:1405–1410
Garden AS, Harris J, Vokes EE, Forastiere AA, Ridge JA, Jones C, Horwitz EM, Glisson BS, Nabell L, Cooper JS, Demas W, Gore E (2004) Preliminary results of radiation therapy oncology group 9703: a randomized phase ii trial of concurrent radiation and chemotherapy for advanced squamous cell carcinomas of the head and neck. J Clin Oncol 22(14):2856–2864
Denis F, Garaud P, Bardet E, Alfonsi M, Sire C, Germain T, Bergerot P, Rhein B, Tortochaux J, Calais G (2004 Jan 1) Final results of the 94-01 French head and neck oncology and radiotherapy group randomized trial comparing radiotherapy alone with concomitant radiochemotherapy in advanced-stage oropharynx carcinoma. Clin Oncol 22(1):69–76 Epub 2003 Dec 2
Machtay M, Moughan J, Trotti A, Garden AS, Weber RS, Cooper JS, Forastiere A, Ang KK (2008) Factors associated with severe late toxicity after concurrent chemoradiation for locally advanced head and neck cancer: an RTOG analysis. J Clin Oncol 26:3582–3589
Hrzenjak A, Moinfar F, Kremser ML, Strohmeier B, Staber PB, Zatloukal K, Denk H (2006) Valproate inhibition of histone deacetylase 2 affects differentiation and decreases proliferation of endometrial stromal sarcoma cells. Mol Cancer Ther 5:2203–2210
Huang BH, Laban M, Leung CH et al (2005) Inhibition of histone deacetylase 2 increases apoptosis and p21Cip1/WAF1 expression, independent of histone deacetylase 1. Cell Death Differ 12:395–404
Westendorf JJ, Zaidi SK, Cascino JE, Kahler R, van Wijnen AJ, Lian JB, Yoshida M, Stein GS, Li X (2002) Runx2 (Cbfa1, AML-3) interacts with histone deacetylase 6 and represses the p21(CIP1/WAF1) promoter. Mol Cell Biol 22:7982–7992
Fraga MF, Ballestar E, Villar-Garea A, Boix-Chornet M, Espada J, Schotta G, Bonaldi T, Haydon C, Ropero S, Petrie K, Iyer NG, Pérez-Rosado A, Calvo E, Lopez JA, Cano A, Calasanz MJ, Colomer D, Piris MÁ, Ahn N, Imhof A, Caldas C, Jenuwein T, Esteller M (2005) Loss of acetylation at Lys16 and trimethylation at Lys20 of histone H4 is a common hallmark of human cancer. Nat Genet 37:391–400
Park SY, Jun JA, Jeong KJ et al (2011) Histone deacetylases 1, 6 and 8 are critical for invasion in breast cancer. Oncol Rep 25:1677–1168
Senese S, Zaragoza K, Minardi S et al (2007) Role for histone deacetylase 1 in human tumor cell proliferation. Mol Cell Biol 27:47844795
Oehme I, Deubzer HE, Wegener D, Pickert D, Linke JP, Hero B, Kopp-Schneider A, Westermann F, Ulrich SM, von Deimling A, Fischer M, Witt O (2009) Histone deacetylase 8 in neuroblastoma tumorigenesis. Clin Cancer Res 15(1):91–99
Haberland M, Montgomery RL, Olson EN (2009) The many roles of histone deacetylases in development and physiology: implications for disease and therapy. Nat Rev Genet 10:32–42
Peng L, Seto E (2011) Deacetylation of nonhistone proteins by HDACs and the implications in cancer. Handb Exp Pharmacol 206:39–56
Marks P, Rifkind RA, Richon VM et al (2001) Histone deacetylases and cancer: causes and therapies. Nat Rev Cancer 1:194–202
Dokmanovic M, Marks PA (2005) Prospects: histone deacetylase inhibitors. J Cell Biochem 96:293–304
Takumi S, Katsuhiro U, Takeshi O et al (2006) Aberrant expression of histone deacetylase 6 in oral squamous cell carcinoma. Int J Oncol 29:117–124
Weidle UH, Grossmann A (2000) Inhibition of histone deacetylases: a new strategy to target epigenetic modifications for anticancer treatment. Anticancer Res 20:1471–1485
Rothgiesser KM, Fey M, Hottiger MO (2010) Acetylation of p65 at lysine 314 is important for late NF-κB-dependent gene expression. BMC Genomics 11:22
Buerki C, Rothgiesser KM, Valovka T, Owen HR, Rehrauer H, Fey M, Lane WS, Hottiger MO (2008) Functional relevance of novel p300-mediated lysine 314 and 315 acetylation of RelA/p65. Nucleic Acids Res 36(5):1665–1680
Sterner DE, Berger SL (2000) Acetylation of histones and transcription-related factors. Microbiol Mol Biol Rev 64:435–459
Rothgiesser KM, Erener S, Waibel S, Luscher B, Hottiger MO (2010) SIRT2 regulates NF-κB dependent gene expression through deacetylation of p65 Lys310. J Cell Sci 123:4251–4258
Lehrmann H, Pritchard LL, Harel-Bellan A (2002) Histone acetyltransferases and deacetylases in the control of cell proliferation and differentiation. Adv Cancer Res 86:41–65
Gregoretti IV, Lee YM, Goodson HV (2004) Molecular evolution of the histone deacetylase family: functional implications of phylogenetic analysis. J Mol Biol 338(1):17–31
Khan O, La Thangue NB (2012) HDAC inhibitors in cancer biology: emerging mechanisms and clinical applications. Immunol Cell Biol 90:85–94
Kim HJ, Bae SC (2011) Histone deacetylase inhibitors: molecular mechanisms of action and clinical trials as anticancer drugs. Am J Transl Res 3(2):166–179
Johnstone RW, Licht JD (2003) Histone deacetylase inhibitors in cancer therapy: is transcription the primary target? Cancer Cell 4(1):13–18
West AC, Johnstone RW (2014) New and emerging HDAC inhibitors for cancer treatment. J Clin Invest 124:30–39
Blumenschein GR, Kies MS, Papadimitrakopoulou VA et al Phase II trial of the histone deacetylase inhibitor vorinostat (Zolinao, SAHA) in patients with recurrent and/or metastatic head and neck cancer. Investig New Drugs 26(1):81–87
Gillenwater AM, Zhong M, Lotan R (2007) Histone deacetylase inhibitor suberoylanilide hydroxamic acid induces apoptosis through both mitochondrial and Fas (Cd95) signaling in head and neck squamous carcinoma cells. Mol Cancer Ther 6(11):2967–2975
Takada Y, Gillenwater A, Ichikawa H, Aggarwal BB (2006) Suberoylanilide hydroxamic acid potentiates apoptosis, inhibits invasion, and abolishes osteoclastogenesis by suppressing nuclear factor-{kappa}B activation. J Biol Chem 281:5612–5622
Bali P, Pranpat M, Swaby R, Fiskus W, Yamaguchi H, Balasis M, Rocha K, Wang HG, Richon V, Bhalla K (2005) Activity of suberoylanilide hydroxamic acid against human breast cancer cells with amplification of her-2. Clin Cancer Res 11(17):6382–6389
Kelly WK, O’Connor OA, Krug LM et al (2005) Phase I study of an oral histone deacetylase inhibitor, suberoylanilide hydroxamic acid, in patients with advanced cancer. J Clin Oncol 23:3923–3931
Kumar B, Yadav A, Lang JC et al (2015) Suberoylanilide hydroxamic acid (SAHA) reverses chemoresistance in head and neck cancer cells by targeting cancer stem cells via the downregulation of nanog. Genes and Cancer 6(3):169–168
Tsai LL, Yu CC, Chang YC et al (2014) Markedly increased Oct4 and Nanog expression correlates with cisplatin resistance in oral squamous cell carcinoma. J Oral Pathol Med 40:621628
Lu X, Mazur SJ, Lin T, Appella E, Xu Y (2014) The pluripotency factor nanog promotes breast cancer tumorigenesis and metastasis. Oncogene 33:2655–2664
Chung YL, Wang AJ, Yao LF (2004) Antitumor histone deacetylase inhibitors suppress cutaneous radiation syndrome: implications for increasing therapeutic gain in cancer radiotherapy. Mol Cancer Ther 3:317–325
Christiansen AJ, West A, Banks KM, Haynes NM, Teng MW, Smyth MJ, Johnstone RW (2011) Eradication of solid tumors using histone deacetylase inhibitors combined with immune-stimulating antibodies. Proc Natl Acad Sci U S A 108:4141–4146
Manning J, Indrova M, Lubyova B, Pribylova H, Bieblova J, Hejnar J, Simova J, Jandlova T, Bubenik J, Reinis M (2008) Induction of MHC class I molecule cell surface expression and epigenetic activation of antigen-processing machinery components in a murine model for human papilloma virus 16-associated tumors. Immunology 123:218–227
Bourhis J, LeMaitre A, Baujat B et al (2007) Individual patients' data meta-analyses in head and neck cancer. Curr Opin Oncol 19:188–194
Murphy BA, Gilbert J, Cmelak A, Ridner SH (2007) Symptom control issues and supportive care of patients with head and neck cancers. Clin Adv Hematol Oncol 5(10):807–822
Trotti A, Bellm LA, Epstein JB, Frame D, Fuchs HJ, Gwede CK, Komaroff E, Nalysnyk L, Zilberberg MD (2003) Mucositis incidence, severity and associated outcomes in patients with head and neck cancer receiving radiotherapy with or without chemotherapy: a systematic literature review. Radiother Oncol 66:253–262
Maesschalck T, Dulguerov N, Caparrotti F et al (2016) Comparison of the incidence of osteoradionecrosis with conventional radiotherapy and intensity-modulated radiotherapy. Head Neck 38:1695–1702
Bishop S, Reed WM (2015) The provision of enteral nutritional support during definitive chemoradiotherapy in head and neck cancer patients. J Med Radiat Sci 62:267–276
Hutcheson KA, Lewin JS, Barringer DA, Lisec A, Gunn GB, Moore MWS, Holsinger FC (2012) Late dysphagia after radiotherapy-based treatment of head and neck cancer. Cancer 118:5793–5799
Ang KK, Zhang Q, Rosenthal DI, Nguyen-Tan PF, Sherman EJ, Weber RS, Galvin JM, Bonner JA, Harris J, el-Naggar AK, Gillison ML, Jordan RC, Konski AA, Thorstad WL, Trotti A, Beitler JJ, Garden AS, Spanos WJ, Yom SS, Axelrod RS (2014) Randomized phase III trial of concurrent accelerated radiation plus cisplatin with or without cetuximab for stage III to IV head and neck carcinoma: RTOG 0522. J Clin Oncol 32:2940–2950
Ramalingam SS, Parise RA, Ramanathan RK et al (2007) Phase I and pharmacokinetic study of vorinostat, a histone deacetylase inhibitor, in combination with carboplatin and paclitaxel for advanced solid malignancies. Clin Cancer Res 13:3605–3610
Haigentz M Jr, Kim M, Sarta C, Lin J, Keresztes RS, Culliney B, Gaba AG, Smith RV, Shapiro GI, Chirieac LR, Mariadason JM, Belbin TJ, Greally JM, Wright JJ, Haddad RI (2012) Phase II trial of the histone deacetylase inhibitor romidepsin in patients with recurrent/metastatic head and neck cancer. Oral Oncol 48:1281–1288
Caponigro F, Di Gennaro E, Ionna F et al (2016) Phase II clinical study of valproic acid plus cisplatin and cetuximab in recurrent and/or metastatic squamous cell carcinoma of head and neck-V-CHANCE trial. BMC Cancer 16(1):918
Kim MS, Blake M, Baek JH, Kohlhagen G, Pommier Y, Carrier F (2003) Inhibition of histone deacetylase increases cytotoxicity to anticancer drugs targeting DNA. Cancer Res 63:7291–7300
Workman JL, Kingston RE (1998) Alteration of nucleosome structure as a mechanism of transcriptional regulation. Annu Rev Biochem 67:545–579
Sato T, Suzuki M, Sato Y, Echigo S, Rikiishi H (2006) Sequence-dependent interaction between cisplatin and histone deacetylase inhibitors in human oral squamous cell carcinoma cells. Int J Oncol 28:1233–1241
Gressette M, Vérillaud B, Jimenez-Pailhès AS, Lelièvre H, Lo KW, Ferrand FR, Gattolliat CH, Jacquet-Bescond A, Kraus-Berthier L, Depil S, Busson P (2014) Treatment of nasopharyngeal carcinoma cells with the histone-deacetylase inhibitor abexinostat: cooperative effects with cis-platin and radiotherapy on patient-derived xenografts. PLoS One 9(3):e91325
Jung M, Kozikowski A, Dritschilo A (2005) Rational design and development of radiation-sensitizing histone deacetylase inhibitors. Chem Biodivers 2:1452–1461
Chinnaiyan P, Vallabhaneni G, Armstrong E, Huang SM, Harari PM (2005) Modulation of radiation response by histone deacetylase inhibition. Int J Radiat Oncol Biol Phys 62(1):223–229
Chen X, Wong P, Radany EH, Stark JM, Laulier C, Wong JYC (2012) Suberoylanilide hydroxamic acid as a radiosensitizer through modulation of RAD51 protein and inhibition of homology-directed repair in multiple myeloma. Mol Cancer Res 10(8):1052–1064
Blattmann C, Oertel S, Ehemann V, Thiemann M, Huber PE, Bischof M, Witt O, Deubzer HE, Kulozik AE, Debus J, Weber KJ (2010) Enhancement of radiation response in osteosarcoma and rhabdomyosarcoma cell lines by histone deacetylase inhibition. Int J Radiat Oncol Biol Phys 78(1):237–245
Chen X, Wong P, Radany E, Wong JYC (2009) HDAC inhibitor, valproic acid, induces p53–dependent radiosensitization of colon cancer cells. Cancer Biother Radiopharm 24(6):689–699
Losson H, Schnekenburger M, Dicato M, et al (2016) Natural compound histone deacetylase inhibitors (HDACi): synergy with inflammatory signaling pathway modulators and clinical applications in cancer. Molecules 21(11)
Wang D, Zhao M, Chen G, Cheng X, Han X, Lin S, Zhang X, Yu X (2013) The histone deacetylase inhibitor vorinostat prevents TNFα-induced necroptosis by regulating multiple signaling pathways. Apoptosis 18(11):1348–1362
Cantley MD, Fairlie DP, Bartold PM, Rainsford KD, le GT, Lucke AJ, Holding CA, Haynes DR (2011) Inhibitors of histone deacetylases in class I and class II suppress human osteoclasts in vitro. J Cell Physiol 226(12):3233–3324
Bruzzese F, Leone A, Rocco M, Carbone C, Piro G, Caraglia M, di Gennaro E, Budillon A (2011) HDAC inhibitor vorinostat enhances the antitumor effect of gefitinib in squamous cell carcinoma of head and neck by modulating ErbB receptor expression and reverting EMT. J Cell Physiol 226(9):2378–2390
Kral AM, Ozerova N, Close J, Jung J, Chenard M, Fleming J, Haines BB, Harrington P, Maclean J, Miller TA, Secrist P, Wang H, Heidebrecht RW Jr (2014) Divergent kinetics differentiate the mechanism of action of two HDAC inhibitors. Biochemistry 53(4):725–734
Rinkel RN, Verdonck-de Leeuw IM, Doornaert P, Buter J, de Bree R, Langendijk JA, Aaronson NK, Leemans CR (2016) Prevalence of swallowing and speech problems in daily life after chemoradiation for head and neck cancer based on cut-off scores of the patient-reported outcome measures SWAL-QOL and SHI. Eur Arch Otorhinolaryngol 273(7):1849–1855
Acknowledgements
This study was approved and funded by the National Comprehensive Cancer Network (NCCN) Oncology Research Program from general research support provided by Merck & Co., Inc.
Funding
This study was funded by the National Comprehensive Cancer Network (NCCN) Oncology Research Program from general research support provided by Merck & Co., Inc.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
All authors declare that there are no conflicts of interest.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Rights and permissions
About this article
Cite this article
Teknos, T.N., Grecula, J., Agrawal, A. et al. A phase 1 trial of Vorinostat in combination with concurrent chemoradiation therapy in the treatment of advanced staged head and neck squamous cell carcinoma. Invest New Drugs 37, 702–710 (2019). https://doi.org/10.1007/s10637-018-0696-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10637-018-0696-4