Summary
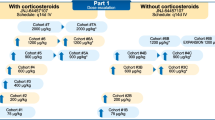
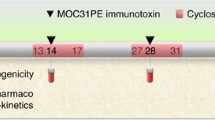
The efficacy of anti-CD33 immunoconjugates had been previously demonstrated for gemtuzumab-ozogamicin. AVE9633 is an anti-CD33-maytansine conjugate created by ImmunoGen Inc. Phase I trials of AVE9633 were performed in patients with AML to evaluate tolerability, pharmacokinetics and pharmacodynamics. Three phase I studies of AVE9633 were performed in 54 patients with refractory/relapsed AML, evaluating drug infusion on day 1 of a 21-day cycle (Day 1 study), day 1 and 8 (Day 1/8 study) and day 1, 4 and 7 (Day 1/4/7 study) of a 28-day cycle. Toxicity was mainly allergic reaction during infusion (3 grade 3 bronchospasms). DLT was reached for the D1–D7 schedule at 150 mg/sqm (1 keratitis, 1 liver toxicity), and the MTD was set at 130 mg/sqm for this schedule. In the two other phases I, the DLT was not reached. In the Day 1/8 study, CD33 on peripheral blasts was saturated and down-modulated for doses of 75 mg/m2 × 2 or higher, which was correlated with WBC kinetics and plasma levels of AVE9633. Decrease of DM4/CD33 ratio on the blasts surface between day 1 and 8 was the rational for evaluating day 1/4/7 schedule. This induced relatively constant DM4/CD33 levels over the first 8 days, however no activity was noted. One CRp, one PR and biological activity in five other patients were observed in this study. The Day 1 and Day 1/4/7 studies were early discontinued because of drug inactivity at doses significantly higher than CD33 -saturating doses. No myelossuppression was observed at any trial of AVE9633. The pharmacokinetics/pharmacodynamics data obtained in these studies will provide very useful information for the design of the next generation of immunoconjugates.



Similar content being viewed by others
References
Shipley JL, Butera JN (2009) Acute myelogenous leukemia. Exp Hematol 37:649–58
Dombret H, Raffoux E, Gardin C (2008) Acute myeloid leukemia in the elderly. Semin Oncol 35:430–8
Advani AS, Hunger SP, Burnett AK (2009) Acute leukemia in adolescents and young adults. Semin Oncol 36(3):213–26
Kreitman RJ (2009) Recombinant immunotoxins for the treatment of chemoresistant hematologic malignancies. Curr Pharm Des 15:2652–64
Legrand O, Perrot JY, Baudard M et al (2000) The immunophenotype of 177 adults with acute myeloid leukemia: proposal of a prognostic score. Blood 96(3):870–7
Sievers EL, Appelbaum FR, Spielberger RT et al (1999) Selective ablation of acute myeloid leukemia using antibody-targeted chemotherapy: a phase I study of an anti-CD33 calicheamicin immunoconjugate. Blood 93:3678–84
Issell BF, Crook ST (1978) Maytansine. Cancer Treat Rev 5:199–207
Kupchan SM, Komoda Y, Court WA et al (1972) Maytansine, a novel antileukemic ansa macrolide from Maytenus ovatus. J Am Chem Soc 94:1354–6
Remillard S, Rebhun LI, Howie GA, Kupchan SM (1975) Antimitotic activity of the potent tumor inhibitor maytansine. Science 189(4207):1002–5
Chari RV, Xie H, Leece BA et al (2005) Preclinical evaluation of an anti-CD33-maytansinoid immunoconjugate, huMy9-6-DM4 (AVE9633) for the targeted therapy of acute myeloid leukemia. AACR; Abst #LB-287
Lin CM, Hanzel E, Wolpert-Defilip MK (1981) Binding of maytansine to tubulin: competition with other mitotic inhibitors. Chem Pathol Pharmacol 31:443–451
Cheson BD, Bennett JM, Kopecky KJ (2003) Revised recommendations of the international working group for diagnosis, standardization of response criteria, treatment outcomes and reporting standards for therapeutic trials in acute myeloid leukemia. J Clin Oncol 24:4642–4649
Erickson HK, Park PU, Widdison WC, Kovtun YV et al (2006) Antibody-maytansinoid conjugates are activated in targeted cancer cells by lysosomal degradation and linker-dependent intracellular processing. Cancer Res 66:4426–4433
Parker SW, Lamri N, Simeoni LA (2003) Analysis of P-glycoprotein-mediated membrane transport in human peripheral blood lymphocytes using the UIC2 shift assay. Cytom A 53:67–78
Tang R, Cohen S, Perrot JY et al (2009) P-gp activity is a critical resistance factor against AVE9633 and DM4 cytotoxicity in leukaemia cell lines, but not a major mechanism of chemoresistance in cells from acute myeloid leukaemia patients. BMC Cancer 9:199–203
Vidriales MB, Orfao A, Lopez-Berges MC et al (1995) Prognostic value of S-phase cells in AML patients. Br J Haematol 89:342–348
Conflicts of interest/disclosure
The authors declare no competing financial interest.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM 1
(DOC 24 kb)
Rights and permissions
About this article
Cite this article
Lapusan, S., Vidriales, M.B., Thomas, X. et al. Phase I studies of AVE9633, an anti-CD33 antibody-maytansinoid conjugate, in adult patients with relapsed/refractory acute myeloid leukemia. Invest New Drugs 30, 1121–1131 (2012). https://doi.org/10.1007/s10637-011-9670-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10637-011-9670-0