Abstract
Purpose
The study evaluated the safety, tolerability, and pharmacokinetics of BMS-936561, a fully human monoclonal antibody-drug conjugate targeting CD70 cell-surface protein.
Methods
Eligible patients had ECOG performance status 0–2 and received ≤3 prior chemotherapy regimens. An initial accelerated titration design enrolling one patient per dose level was followed by 3 + 3 dose escalation with the first observation of a grade ≥2 adverse event (AE). We tested escalating doses of BMS-936561 (0.5, 1, 2, 4, 8, 15 mg/kg) administered every 21 days in a 42 day cycle for a maximum of 17 cycles. Pharmacokinetic samples were collected in cycle 1.
Results
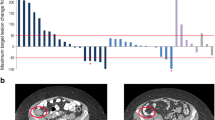
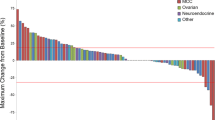
A total of 26 patients enrolled; 16 and 10 for the escalation and expansion cohorts, respectively. Median age was 63 years (48–74); 18 males and 25 Caucasians. There was no defined MTD per protocol, but a DLT of grade 3 hypersensitivity was recorded in 2 of 16 (13 %) subjects at the highest dose of 15 mg/kg. The most frequent AEs were: fatigue (85 %), nausea (54 %), and decreased appetite (39 %). Delayed toxicities (facial edema and pleural/pericardial effusions) occurred in 6 of 16 (38 %) subjects at the 15 mg/kg dose. PK analysis showed a dose-proportional increase in active drug levels with increasing doses. There was disease stabilization in 18 of 26 patients (69 %) without correlation with received dose.
Conclusions
BMS-936561 is well tolerated over a wide range of doses in patients with advanced ccRCC and B-NHL. The 8 mg/kg dose was the highest best tolerated dose and the recommended dose for future studies.
Similar content being viewed by others
References
McEarchern JA, Smith LM, McDonagh CF, Klussman K, Gordon KA, Morris-Tilden CA, Duniho S, Ryan M, Boursalian TE, Carter PJ, Grewal IS, Law CL (2008) Preclinical characterization of SGN-70, a humanized antibody directed against CD70. Clin Cancer Res 14(23):7763–7772. doi:10.1158/1078-0432.CCR-08-0493
Teicher BA, Chari RV (2011) Antibody conjugate therapeutics: challenges and potential. Clin Cancer Res 17(20):6389–6397. doi:10.1158/1078-0432.CCR-11-1417
McEarchern JA, Oflazoglu E, Francisco L, McDonagh CF, Gordon KA, Stone I, Klussman K, Turcott E, van Rooijen N, Carter P, Grewal IS, Wahl AF, Law CL (2007) Engineered anti-CD70 antibody with multiple effector functions exhibits in vitro and in vivo antitumor activities. Blood 109(3):1185–1192. doi:10.1182/blood-2006-07-034017
Thevanayagam L, Bell A, Chakraborty I, Sufi B, Gangwar S, Zang A, Rangan V, Rao C, Wang Z, Pan C, Chong C, Cardarelli P, Deshpande S, Srinivasan M (2013) Novel detection of DNA-alkylated adducts of antibody-drug conjugates with potentially unique preclinical and biomarker applications. Bioanalysis 5(9):1073–1081. doi:10.4155/bio.13.57
Bowman MR, Crimmins MA, Yetz-Aldape J, Kriz R, Kelleher K, Herrmann S (1994) The cloning of CD70 and its identification as the ligand for CD27. J Immunol 152(4):1756–1761
Borst J, Hendriks J, Xiao Y (2005) CD27 and CD70 in T cell and B cell activation. Curr Opin Immunol 17(3):275–281. doi:10.1016/j.coi.2005.04.004
Nilsson A, de Milito A, Mowafi F, Winberg G, Bjork O, Wolpert EZ, Chiodi F (2005) Expression of CD27-CD70 on early B cell progenitors in the bone marrow: implication for diagnosis and therapy of childhood ALL. Exp Hematol 33(12):1500–1507. doi:10.1016/j.exphem.2005.10.005
Adam PJ, Terrett JA, Steers G, Stockwin L, Loader JA, Fletcher GC, Lu LS, Leach BI, Mason S, Stamps AC, Boyd RS, Pezzella F, Gatter KC, Harris AL (2006) CD70 (TNFSF7) is expressed at high prevalence in renal cell carcinomas and is rapidly internalised on antibody binding. Br J Cancer 95(3):298–306. doi:10.1038/sj.bjc.6603222
Junker K, Hindermann W, von Eggeling F, Diegmann J, Haessler K, Schubert J (2005) CD70: a new tumor specific biomarker for renal cell carcinoma. J Urol 173(6):2150–2153. doi:10.1097/01.ju.0000158121.49085.ba
Law CL, Gordon KA, Toki BE, Yamane AK, Hering MA, Cerveny CG, Petroziello JM, Ryan MC, Smith L, Simon R, Sauter G, Oflazoglu E, Doronina SO, Meyer DL, Francisco JA, Carter P, Senter PD, Copland JA, Wood CG, Wahl AF (2006) Lymphocyte activation antigen CD70 expressed by renal cell carcinoma is a potential therapeutic target for anti-CD70 antibody-drug conjugates. Cancer Res 66(4):2328–2337. doi:10.1158/0008-5472.CAN-05-2883
Grewal IS (2008) CD70 as a therapeutic target in human malignancies. Expert Opin Ther Targets 12(3):341–351. doi:10.1517/14728222.12.3.341
McDonagh CF, Kim KM, Turcott E, Brown LL, Westendorf L, Feist T, Sussman D, Stone I, Anderson M, Miyamoto J, Lyon R, Alley SC, Gerber HP, Carter PJ (2008) Engineered anti-CD70 antibody-drug conjugate with increased therapeutic index. Mol Cancer Ther 7(9):2913–2923. doi:10.1158/1535-7163.MCT-08-0295
Oflazoglu E, Stone IJ, Gordon K, Wood CG, Repasky EA, Grewal IS, Law CL, Gerber HP (2008) Potent anticarcinoma activity of the humanized anti-CD70 antibody h1F6 conjugated to the tubulin inhibitor auristatin via an uncleavable linker. Clin Cancer Res 14(19):6171–6180. doi:10.1158/1078-0432.CCR-08-0916
Boger DL, Johnson DS, Yun W, Tarby CM (1994) Molecular basis for sequence selective DNA alkylation by (+)- and ent-(−)-CC-1065 and related agents: alkylation site models that accommodate the offset AT-rich adenine N3 alkylation selectivity. Bioorgan Med Chem 2(2):115–135
Gomi K, Kobayashi E, Miyoshi K, Ashizawa T, Okamoto A, Ogawa T, Katsumata S, Mihara A, Okabe M, Hirata T (1992) Anticellular and antitumor activity of duocarmycins, novel antitumor antibiotics. Jpn J Cancer Res 83(1):113–120
Alberts SR, Suman VJ, Pitot HC, Camoriano JK, Rubin J (2007) Use of KW-2189, a DNA minor groove-binding agent, in patients with hepatocellular carcinoma: a north central cancer treatment group (NCCTG) phase II clinical trial. J Gastrointest Cancer 38(1):10–14
Markovic SN, Suman VJ, Vukov AM, Fitch TR, Hillman DW, Adjei AA, Alberts SR, Kaur JS, Braich TA, Leitch JM, Creagan ET (2002) Phase II trial of KW2189 in patients with advanced malignant melanoma. Am J Clin Oncol 25(3):308–312
Small EJ, Figlin R, Petrylak D, Vaughn DJ, Sartor O, Horak I, Pincus R, Kremer A, Bowden C (2000) A phase II pilot study of KW-2189 in patients with advanced renal cell carcinoma. Invest New Drugs 18(2):193–197
Schwartz GH, Patnaik A, Hammond LA, Rizzo J, Berg K, Von Hoff DD, Rowinsky EK (2003) A phase I study of bizelesin, a highly potent and selective DNA-interactive agent, in patients with advanced solid malignancies. Ann Oncol 14(5):775–782
Wang H, Rangan VS, Sung MC, Passmore D, Kempe T, Wang X, Thevanayagam L, Pan C, Rao C, Srinivasan M, Zhang Q, Gangwar S, Deshpande S, Cardarelli P, Marathe P, Yang Z (2015) Pharmacokinetic characterization of BMS-936561, an anti-CD70 antibody-drug conjugate, in preclinical animal species and prediction of its pharmacokinetics in humans. Biopharm Drug Dispos. doi:10.1002/bdd.1953
Verma S, Miles D, Gianni L, Krop IE, Welslau M, Baselga J, Pegram M, Oh DY, Dieras V, Guardino E, Fang L, Lu MW, Olsen S, Blackwell K (2012) Trastuzumab emtansine for HER2-positive advanced breast cancer. N Engl J Med 367(19):1783–1791. doi:10.1056/NEJMoa1209124
Younes A, Gopal AK, Smith SE, Ansell SM, Rosenblatt JD, Savage KJ, Ramchandren R, Bartlett NL, Cheson BD, de Vos S, Forero-Torres A, Moskowitz CH, Connors JM, Engert A, Larsen EK, Kennedy DA, Sievers EL, Chen R (2012) Results of a pivotal phase II study of brentuximab vedotin for patients with relapsed or refractory Hodgkin’s lymphoma. J Clin Oncol 30(18):2183–2189. doi:10.1200/JCO.2011.38.0410
Gopal AK, Chen R, Smith SE, Ansell SM, Rosenblatt JD, Savage KJ, Connors JM, Engert A, Larsen EK, Chi X, Sievers EL, Younes A (2015) Durable remissions in a pivotal phase 2 study of brentuximab vedotin in relapsed or refractory Hodgkin lymphoma. Blood 125(8):1236–1243. doi:10.1182/blood-2014-08-595801
Mack F, Ritchie M, Sapra P (2014) The next generation of antibody drug conjugates. Semin Oncol 41(5):637–652. doi:10.1053/j.seminoncol.2014.08.001
Wang J, Song P, Schrieber S, Liu Q, Xu Q, Blumenthal G, Amiri Kordestani L, Cortazar P, Ibrahim A, Justice R, Wang Y, Tang S, Booth B, Mehrotra N, Rahman A (2014) Exposure-response relationship of T-DM1: insight into dose optimization for patients with HER2-positive metastatic breast cancer. Clin Pharmacol Ther 95(5):558–564. doi:10.1038/clpt.2014.24
Gorovits B, Alley SC, Bilic S, Booth B, Kaur S, Oldfield P, Purushothama S, Rao C, Shord S, Siguenza P (2013) Bioanalysis of antibody-drug conjugates: American association of pharmaceutical scientists antibody-drug conjugate working group position paper. Bioanalysis 5(9):997–1006. doi:10.4155/bio.13.38
Acknowledgments
We thank patients and their families for participating in the study.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Parul Gulati, Aadhar Shah, Christoph Matthias Ahlers, Pina M. Cardarelli and Lewis J. Cohen are employees of Bristol-Myers Squibb.Taofeek K. Owonikoko received research funding from Bristol-Myers Squibb.
Rights and permissions
About this article
Cite this article
Owonikoko, T.K., Hussain, A., Stadler, W.M. et al. First-in-human multicenter phase I study of BMS-936561 (MDX-1203), an antibody-drug conjugate targeting CD70. Cancer Chemother Pharmacol 77, 155–162 (2016). https://doi.org/10.1007/s00280-015-2909-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00280-015-2909-2