Abstract
Introduction
Leber Congenital Amaurosis (LCA) is an inherited retinal disease that presents in infancy with severely decreased vision, nystagmus, and extinguished electroretinography findings. LCA8 is linked to variants in the Crumbs homolog 1 (CRB1) gene.
Case Description
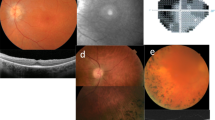
We report a novel CRB1 variant in a 14-year-old male presenting with nystagmus, worsening vision, and inability to fixate on toys in his infancy. Color fundus photography revealed nummular pigments in the macula and periphery. Imaging studies revealed thickened retina on standard domain optical coherence tomography and widespread atrophy of the retinal pigment epithelium on autofluorescence. Full-field electroretinography revealed extinguished scotopic and significantly reduced photopic responses. Genetic testing demonstrated a novel homozygous variant, c.3057 T > A; p.(Tyr1019Ter), in the CRB1 gene. This variant is not currently amenable to base editing, however, in silico analysis revealed several potential prime editing strategies for correction.
Conclusion
This case presentation is consistent with LCA8, suggesting pathogenicity of this novel variant and expanding our knowledge of disease-causing CRB1 variants.




Similar content being viewed by others
References
Kumaran N et al (2017) Leber congenital amaurosis/early-onset severe retinal dystrophy: clinical features, molecular genetics and therapeutic interventions. Br J Ophthalmol 101(9):1147–1154
Koenekoop RK (2004) An overview of Leber congenital amaurosis: a model to understand human retinal development. Surv Ophthalmol 49(4):379–398
den Hollander AI et al (2008) Leber congenital amaurosis: genes, proteins and disease mechanisms. Prog Retin Eye Res 27(4):391–419
Bujakowska K et al (2012) CRB1 mutations in inherited retinal dystrophies. Hum Mutat 33(2):306–315
Tsang SH, Sharma T (2018) Leber congenital amaurosis. Adv Exp Med Biol 1085:131–137
Abouzeid H et al (2006) A G1103R mutation in CRB1 is co-inherited with high hyperopia and Leber congenital amaurosis. Ophthalmic Genet 27(1):15–20
Costa BLD et al (2023) Clinical and therapeutic evaluation of the ten most prevalent CRB1 mutations. Biomedicines. 11(2):385
Tsang SH et al (2014) Whole exome sequencing identifies CRB1 defect in an unusual maculopathy phenotype. Ophthalmology 121(9):1773–1782
Tosi J et al (2009) Case report: autofluorescence imaging and phenotypic variance in a sibling pair with early-onset retinal dystrophy due to defective CRB1 function. Curr Eye Res 34(5):395–400
Richards S et al (2015) Standards and guidelines for the interpretation of sequence variants: a joint consensus recommendation of the American college of medical genetics and genomics and the association for molecular pathology. Genet Med 17(5):405–424
Simonelli F et al (2007) Clinical and molecular genetics of Leber’s congenital amaurosis: a multicenter study of Italian patients. Invest Ophthalmol Vis Sci 48(9):4284–4290
den Hollander AI et al (2001) CRB1 has a cytoplasmic domain that is functionally conserved between human and Drosophila. Hum Mol Genet 10(24):2767–2773
Benayoun L et al (2009) Genetic heterogeneity in two consanguineous families segregating early onset retinal degeneration: the pitfalls of homozygosity mapping. Am J Med Genet A 149A(4):650–656
Varela MD et al (2023) CRB1-associated retinal dystrophies: genetics, clinical characteristics, and natural history. Am J Ophthalmol 246:107–121
Talib M et al (2017) Genotypic and phenotypic characteristics of CRB1-associated retinal dystrophies: a long-term follow-up study. Ophthalmology 124(6):884–895
Pellissier LP et al (2015) Gene therapy into photoreceptors and Muller glial cells restores retinal structure and function in CRB1 retinitis pigmentosa mouse models. Hum Mol Genet 24(11):3104–3118
Buck TM et al (2021) AAV-CRB2 protects against vision loss in an inducible CRB1 retinitis pigmentosa mouse model. Mol Ther Methods Clin Dev 20:423–441
Boon N et al (2021) Defining phenotype, tropism, and retinal gene therapy using adeno-associated viral vectors (aavs) in new-born brown norway rats with a spontaneous mutation in crb1. Int J Mol Sci 22(7):3563
Boon N et al (2023) AAV-mediated gene augmentation therapy of CRB1 patient-derived retinal organoids restores the histological and transcriptional retinal phenotype. Stem Cell Reports 18(6):1388
Costa BLD et al (2023) Analysis of CRB1 Pathogenic Variants Correctable with CRISPR Base and Prime Editing. Adv Exp Med Biol 1415:103–107
Gao Z, Herrera-Carrillo E, Berkhout B (2018) Delineation of the exact transcription termination signal for type 3 polymerase III. Mol Ther Nucleic Acids 10:36–44
Mairhofer J et al (2015) Preventing T7 RNA polymerase read-through transcription-a synthetic termination signal capable of improving bioprocess stability. ACS Synth Biol 4(3):265–273
Song H, Kang C (2001) Sequence-specific termination by T7 RNA polymerase requires formation of paused conformation prior to the point of RNA release. Genes Cells 6(4):291–301
Jeng ST, Gardner JF, Gumport RI (1990) Transcription termination by bacteriophage T7 RNA polymerase at rho-independent terminators. J Biol Chem 265(7):3823–3830
Chen PJ et al (2021) Enhanced prime editing systems by manipulating cellular determinants of editing outcomes. Cell 184(22):5635-5652e29
McCulloch DL et al (2015) ISCEV Standard for full-field clinical electroretinography (2015 update). Doc Ophthalmol 130(1):1–12
Acknowledgements
We thank the Jonas Children’s Vision Care (JCVC) team and Quinn Lab members for their support and comradery.
Funding
B.L.D.C. is a recipient of the Capes PhD scholarship. S.H.T. and Jonas Children's Vision Care is supported by the National Institute of Health 5P30CA013696, U01 EY030580, U54OD020351, R24EY028758, R24EY027285, 5P30EY019007, R01EY018213, R01EY024698, R01EY026682, R21AG050437, the Schneeweiss Stem Cell Fund, New York State [SDHDOH01-C32590GG-3450000], the Foundation Fighting Blindness New York Regional Research Center Grant [TA-NMT-0116–0692-COLU], Nancy & Kobi Karp, the Crowley Family Funds, The Rosenbaum Family Foundation, Alcon Research Institute, the Gebroe Family Foundation, the Research to Prevent Blindness (RPB) Physician-Scientist Award, unrestricted funds from RPB, New York, NY, USA. P.M.J.Q. is supported by the Curing Retinal Blindness Foundation (CRBF), a New York Stem Cell Foundation (NYSCF)—Druckenmiller Fellowship, and by the National Eye Institute, National Institutes of Health, through Grant Number R01EY034952.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
S.H.T receives financial support from Abeona Therapeutics, Inc and Emendo and is on the scientific and clinical advisory board for Nanoscope Therapeutics. Columbia University has filed patent applications related to CRB1 for which B.L.D.C, S.H.T, and P.M.J.Q are listed as inventors. M.S., M.K. and I.H.M. have no conflicting interests.
Ethical approval
The study was conducted under the Columbia University Institutional Review Board-approved protocol IRB AAAF1849. All procedures were performed in compliance with the tenets of the Declaration of Helsinki. Informed consent was waived due to the retrospective nature of the study and the minimal risk conferred to patients as per the Columbia University Institutional Review Board-approved protocol AAAR8743.
Statement of human rights
The study was conducted under the Columbia University Institutional Review Board-approved protocol IRB AAAF1849. All procedures were performed in compliance with the tenets of the Declaration of Helsinki.
Statement on the welfare of animals
No animals were used in this study.
Informed consent
Informed consent was waived due to the retrospective nature of the study and the minimal risk conferred to patients as per the Columbia University Institutional Review Board-approved protocol AAAR8743.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Sylla, M.M., Kolesinkova, M., da Costa, B.L. et al. A novel pathogenic CRB1 variant presenting as Leber Congenital Amaurosis 8 and evaluation of gene editing feasibility. Doc Ophthalmol 147, 217–224 (2023). https://doi.org/10.1007/s10633-023-09951-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10633-023-09951-w