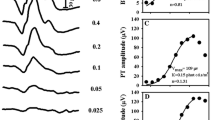
Abstract
Purpose
To analyze the effects of different methods of measurement on the photopic negative response (PhNR), recorded as part of a standard ISCEV Photopic 3.0 ERG responses from patients with a variety of clinical diagnoses.
Methods
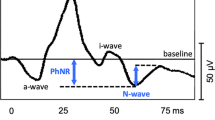
ERGs were recorded from both eyes of 97 patients (187 eyes) as part of a standard clinical assessment. The average age was 56.4 ± 15.7 years, the gender balance was 35 M, 62F, and only recordable responses of acceptable quality were included. PhNR was measured at an identifiable trough before (PhNR1) and after the i-wave (PhNR2), and the amplitudes and peak times were compared with a-, b- and i-wave corresponding parameters. PhNR components were measured: from baseline and from b-wave peak. Correlation between PhNR troughs and with ERG parameters were tested for right eyes. The possibility to predict and substitute PhNR2 amplitude from PhNR1 amplitude was also tested.
Results
PhNR1 was recordable in 97.3% of eyes and PhNR2 in 85.6%. An identifiable PhNR2 peak was found to occur before 65 ms at ~ 50% of the records, while in ~ 38% of the cases was within 65–75 ms in ~ 12%—after 75 ms. The correlation between the PhNR1 and PhNR2 peaks was quite strong (with coefficients 0.81–0.98, depending on method of measurement, and slopes close to 1). The average difference between predicted and measured PhNR2 was reasonably small in absolute (< 2 µV) and relative (< 2%) terms. The correlations between PhNR amplitudes and other ERG component amplitudes showed different ranges of correlation coefficients depending on the method of measurement: for the a-wave amplitudes the range of coefficients was 0.48–0.73, while for the b-wave amplitudes it was 0.30–0.95 and 0.39–0.65 for i-wave.
Conclusion
The strong correlation between the two PhNR troughs could allow using PhNR1 when PhNR2 is poorly defined due to artifacts. Different methods of PhNR measurement lead to different correlations with other ERG parameters, and this needs to be considered when analyzing and comparing PhNR data between studies.





Similar content being viewed by others
Notes
The H35 is broadly defined category which includes nine distinct subcategories (n = 38). The distribution of subcategories and the number of patients within each subcategory were as follows: H35/H53.0 (n = 4); H35.1 (n = 1), H35.3 (n = 13), H35.4 (n = 3), H35.5 (n = 13), H35.8 (n = 3). The full list of all subcategories can be found in [45] and other sources.
The categories as per ICD-10 classification are as follows: 1) H30 or H31; 2) H35 (or 362); 3) H40; 4) H53 (or 368); 5) H40; 6) H47; 7) other (includes G11.8, H44, Z79 and D31).
References
Marmor MF, Arden GB, Nilsson SEG, Zrenner E (1989) Standard for clinical electroretinography. International Standardization Committee. Arch Ophthalmol 107(6):816–819
McCulloch DL et al (2015) ISCEV standard for full-field clinical electroretinography (2015 update). Doc Ophthalmol 130(1):1–12
Sieving PA, Murayama K, Naarendorp F (1994) Push-pull model of the primate photopic electroretinogram: a role for hyperpolarizing neurons in shaping the b-wave. Vis Neurosci 11(3):519–532
Stockton RA, Slaughter MM (1989) B-wave of the electroretinogram: a reflection of ON bipolar cell activity. J Gen Physiol 93(1):101–122
Frishman L et al (2018) ISCEV extended protocol for the photopic negative response (PhNR) of the full-field electroretinogram. Doc Ophthalmol 136(3):207–211
Nagata M (1963) Studies on the photopic ERG of the human retina. Jpn J Ophthalmol 7:96–124
Seiple W, Holopigian K (1993) The ‘OFF’ response of the human electroretinogram does not contribute to the brief flash ‘b-wave’. Vis Neurosci 11:667–673
Rousseau S, McKerral M, Lachapelle P (1996) The i-wave: bridging flash and pattern electroretinography. Electroencephalogr Clin Neurophysiol Suppl 46:165–171
Rufiange M et al (2002) Cone-dominated ERG luminance-response function: the Photopic Hill revisited. Doc Ophthalmol 104(3):231–248
Lachapelle P et al (1998) Evidence supportive of a functional discrimination between photopic oscillatory potentials as revealed with cone and rod mediated retinopathies. Doc Ophthalmol 95(1):35–54
Rosolen SG et al (2004) Comparing the photopic ERG i-wave in different species. Vet Ophthalmol 7(3):189–192
Rangaswamy NV et al (2004) Photopic ERGs in patients with optic neuropathies: comparison with primate ERGs after pharmacologic blockade of inner retina. Invest Ophthalmol Vis Sci 45(10):3827–3837
Viswanathan S et al (1999) The photopic negative response of the macaque electroretinogram: reduction by experimental glaucoma. Invest Ophthalmol Vis Sci 40(6):1124–1136
Colotto A et al (2000) Photopic negative response of the human ERG: losses associated with glaucomatous damage. Invest Ophthalmol Vis Sci 41(8):2205–2211
Viswanathan S et al (2001) The photopic negative response of the flash electroretinogram in primary open angle glaucoma. Invest Ophthalmol Vis Sci 42(2):514–522
Wakili N et al (2008) The photopic negative response of the blue-on-yellow flash-electroretinogram in glaucomas and normal subjects. Doc Ophthalmol 117(2):147–154
Machida S et al (2008) Correlation between photopic negative response and retinal nerve fiber layer thickness and optic disc topography in glaucomatous eyes. Invest Ophthalmol Vis Sci 49(5):2201–2207
Sustar M, Cvenkel B, Brecelj J (2009) The effect of broadband and monochromatic stimuli on the photopic negative response of the electroretinogram in normal subjects and in open-angle glaucoma patients. Doc Ophthalmol 118(3):167–177
North RV et al (2010) Electrophysiological evidence of early functional damage in glaucoma and ocular hypertension. Invest Ophthalmol Vis Sci 51(2):1216–1222
Kim HD, Park JY, Ohn YH (2010) Clinical applications of photopic negative response (PhNR) for the treatment of glaucoma and diabetic retinopathy. Korean J Ophthalmol 24(2):89–95
Kremers J et al (2012) Spectral characteristics of the PhNR in the full-field flash electroretinogram of normals and glaucoma patients. Doc Ophthalmol 124(2):79–90
Huang L et al (2012) Clinical application of photopic negative response of the flash electroretinogram in primary open-angle Glaucoma. Eye Sci 27(3):113–118
Preiser D et al (2013) Photopic negative response versus pattern electroretinogram in early glaucoma. Invest Ophthalmol Vis Sci 54(2):1182–1191
Niyadurupola N et al (2013) Intraocular pressure lowering is associated with an increase in the photopic negative response (PhNR) amplitude in glaucoma and ocular hypertensive eyes. Invest Ophthalmol Vis Sci 54(3):1913–1919
Kirkiewicz M, Lubinski W, Penkala K (2016) Photopic negative response of full-field electroretinography in patients with different stages of glaucomatous optic neuropathy. Doc Ophthalmol 132(1):57–65
Cvenkel B, Sustar M, Perovsek D (2017) Ganglion cell loss in early glaucoma, as assessed by photopic negative response, pattern electroretinogram, and spectral-domain optical coherence tomography. Doc Ophthalmol 135(1):17–28
Kizawa J et al (2006) Changes of oscillatory potentials and photopic negative response in patients with early diabetic retinopathy. Jpn J Ophthalmol 50(4):367–373
Chen H et al (2008) The photopic negative response of flash ERG in nonproliferative diabetic retinopathy. Doc Ophthalmol 117(2):129–135
McFarlane M et al (2012) Blue flash ERG PhNR changes associated with poor long-term glycemic control in adolescents with type 1 diabetes. Invest Ophthalmol Vis Sci 53(2):741–748
Gotoh Y, Machida S, Tazawa Y (2004) Selective loss of the photopic negative response in patients with optic nerve atrophy. Arch Ophthalmol 122(3):341–346
Chen H et al (2006) The photopic negative response of the flash electroretinogram in retinal vein occlusion. Doc Ophthalmol 113(1):53–59
Moon CH et al (2013) Visual prognostic value of photopic negative response and optical coherence tomography in central retinal vein occlusion after anti-VEGF treatment. Doc Ophthalmol 126(3):211–219
Gardasevic Topcic I et al (2014) Morphological and electrophysiological outcome in prospective intravitreal bevacizumab treatment of macular edema secondary to central retinal vein occlusion. Doc Ophthalmol 129(1):27–38
Abed E et al (2015) Functional loss of the inner retina in childhood optic gliomas detected by photopic negative response. Invest Ophthalmol Vis Sci 56(4):2469–2474
Falsini B et al (2016) Nerve growth factor improves visual loss in childhood optic gliomas: a randomized, double-blind, phase II clinical trial. Brain 139(Pt 2):404–414
Moss HE, Park JC, McAnany JJ (2015) The photopic negative response in idiopathic intracranial hypertension. Invest Ophthalmol Vis Sci 56(6):3709–3714
Park JC, Moss HE, McAnany JJ (2018) Electroretinography in idiopathic intracranial hypertension: comparison of the pattern ERG and the photopic negative response. Doc Ophthalmol 136(1):45–55
Wang J et al (2012) The photopic negative response of the flash electroretinogram in multiple sclerosis. Invest Ophthalmol Vis Sci 53(3):1315–1323
Johnson MA, Massof RW (1982) The photomyoclonic reflex: an artefact in the clinical electroretinogram. Br J Ophthalmol 66(6):368–378
Thompson DA et al (2011) Altered electroretinograms in patients with KCNJ10 mutations and EAST syndrome. J Physiol 589(Pt 7):1681–1689
Mortlock KE et al (2010) Inter-subject, inter-ocular and inter-session repeatability of the photopic negative response of the electroretinogram recorded using DTL and skin electrodes. Doc Ophthalmol 121(2):123–134
Binns AM, Mortlock KE, North RV (2011) The relationship between stimulus intensity and response amplitude for the photopic negative response of the flash electroretinogram. Doc Ophthalmol 122(1):39–52
Tang J et al (2014) The test–retest reliability of the photopic negative response (PhNR). Transl Vis Sci Technol 3(6):1
Abed E et al (2018) Early impairment of the full-field photopic negative response in patients with Stargardt disease and pathogenic variants of the ABCA4 gene. Clin Exp Ophthalmol 46(5):519–530
Reference, I.-.-C.P.M.C. ICD10Data. 2018 [cited 2018 9/22/2018]; 2018 ICD-10-CM/PCS Medical Coding Reference]. Available from: https://www.icd10data.com/
Gowrisankaran S et al (2011) Structural and functional measures of inner retinal integrity following visual acuity improvement in a patient with hereditary motor and sensory neuropathy type VI. Ophthalmic Genet 32(3):188–192
Moon CH et al (2011) The time course of visual field recovery and changes of retinal ganglion cells after optic chiasmal decompression. Invest Ophthalmol Vis Sci 52(11):7966–7973
Wu Z et al (2016) Measuring the photopic negative response: viability of skin electrodes and variability across disease severities in glaucoma. Transl Vis Sci Technol 5(2):13
Miyata K et al (2007) Reduction of oscillatory potentials and photopic negative response in patients with autosomal dominant optic atrophy with OPA1 mutations. Invest Ophthalmol Vis Sci 48(2):820–824
Bhatti T et al (2017) Relative genetic and environmental contributions to variations in human retinal electrical responses quantified in a twin study. Ophthalmology 124(8):1175–1185
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no potential conflicts of interest related to this work.
Statement of human rights
The study followed the tenets of the Declaration of Helsinki and was approved by a University of South Florida institutional review board.
Statement on the welfare of animals
No animals were involved in this research.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. The research protocol was approved by the local Institutional Review Board (protocol # Pro00026623).
Informed consent
For this type of study, formal consent is not required.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Ortiz, G., Drucker, D., Hyde, C. et al. The photopic negative response of the Light-adapted 3.0 ERG in clinical settings. Doc Ophthalmol 140, 115–128 (2020). https://doi.org/10.1007/s10633-019-09723-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10633-019-09723-5