Abstract
Background
Average-risk women aged 50–59 years have a lower incidence and mortality of colorectal cancer relative to age-matched men, calling into question the benefit of screening colonoscopy in this age group.
Aims
We aimed to determine whether FOBT is an effective initial screening test in 50–59-year-old women.
Methods
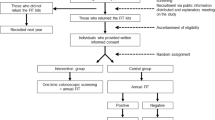
We conducted a cross-sectional study using a computerized endoscopic report generator. We identified 320,906 individuals who had average-risk screening colonoscopy and 32,369 who had colonoscopy for positive FOBT. The primary outcome was the positive predictive value (PPV) of FOBT for large polyp(s) greater than 9 mm, as a surrogate for advanced neoplasia.
Results
Among patients aged 50–59 years undergoing screening colonoscopy, men were more likely than women to have large polyps (6.3 vs 4.2%, p < 0.0001). Black women undergoing screening colonoscopy had higher rates of large polyps compared to non-Black women. The PPV in FOBT-positive men aged 50–54 (11.5%) and 55–59 (14.4%) was higher than in women aged 50–54 (6.1%) and 55–59 (5.4%). Despite this lower PPV, women aged 50–54 with a positive FOBT had a similar rate of large polyps as 50–54-year-old men undergoing screening colonoscopy (6.1 vs 6.3%, p = 0.626).
Conclusions
CRC screening with FOBT identifies 50–59-year-old men and women with a higher risk of large polyps. Since younger women have a lower risk of large polyps than men, screening with FOBT in 50–59-year-old non-Black women could be an effective screening strategy, with outcomes similar to the use of screening colonoscopy in 50–59-year-old men.
Similar content being viewed by others
References
Siegel RL, Miller KD, Jemal A. Cancer statistics. CA Cancer J Clin. 2016;66:7–30.
American Cancer Society. Cancer Facts and Figures 2016. http://www.cancer.org/acs/groups/content/@research/documents/document/acspc-047079.pdf. Accessed January 1, 2016.
Levin B, Lieberman DA, McFarland B, et al. Screening and surveillance for early detection of colorectal cancer and adenomatous polyps, 2008: a joint guideline from the American Cancer Society, the US Multi-Society Task Force on Colorectal Cancer, and the American College of Radiology. Gastroenterology. 2008;134:1570–1595.
Rex DK, Johnson DA, Anderson JC, et al. American College of Gastroenterology guidelines for colorectal cancer screening 2008. Am J Gastroenterol. 2009;104:739–750.
Qaseem A, Denberg TD, Hopkins RH Jr, et al. Screening for colorectal cancer: a guidance statement from the American College of Physicians. Ann Intern Med. 2012;156:378–386.
US Preventive Services Task Force, Bibbins-Domingo K, Grossman DC, et al. Screening for colorectal cancer: US preventive services task force recommendation statement. JAMA. 2016;315:2564–2575.
Klabunde CN, Joseph DA, King JB, et al. Vital signs: colorectal cancer screening test use—United States, 2012. MMWR Morb Mortal Wkly Rep. 2013;62:881–888.
Shapiro JA, Klabunde CN, Thompson TD, et al. Patterns of colorectal cancer test use, including CT colonography, in the 2010 National Health Interview Survey. Cancer Epidemiol Biomark Prev. 2012;21:895–904.
SEER*Explorer. National Cancer Institute. https://seer.cancer.gov/explorer/. Updated April 14, 2017. Accessed April 23, 2017.
Regula J, Rupinski M, Kraszewska E, et al. Colonoscopy in colorectal-cancer screening for detection of advanced neoplasia. N Engl J Med. 2006;355:1863–1872.
Schoenfeld P, Cash B, Flood A, et al. Colonoscopic screening of average-risk women for colorectal neoplasia. N Engl J Med. 2005;352:2061–2068.
Lieberman DA, Williams JL, Holub JL, et al. Race, ethnicity, and sex affect risk for polyps > 9 mm in average-risk individuals. Gastroenterology. 2014;147:351–358.
Ferlitsch M, Reinhart K, Pramhas S, et al. Sex-specific prevalence of adenomas, advanced adenomas and colorectal cancer in individuals undergoing screening colonoscopy. JAMA. 2011;306:1352–1358.
Mandel JS, Bond JH, Church TR, et al. Reducing mortality from colorectal cancer by screening for fecal occult blood. Minnesota Colon Cancer Control Study. N Engl J Med. 1993;328:1365–1371.
Hardcastle JD, Chamberlain JO, Robinson MH, et al. Randomised controlled trial of faecal-occult-blood screening for colorectal cancer. Lancet. 1996;348:1472–1477.
Kronborg O, Fenger C, Olsen J, et al. Randomised study of screening for colorectal cancer with faecal-occult-blood test. Lancet. 1996;348:1467–1471.
Winawer SJ, Zauber AG, Ho MN, et al. Prevention of colorectal cancer by colonoscopic polypectomy. N Engl J Med. 1993;329:1977–1981.
Sonnenberg A, Amorosi SL, Lacey MJ, et al. Patterns of endoscopy in the United States: analysis of data from the Centers for Medicare and Medicaid Services and the National Endoscopic Database. Gastrointest Endosc. 2008;67:489–496.
Lieberman D, Moravec M, Holub J, et al. Polyp size and advanced histology in patients undergoing colonoscopy screening: implications for CT Colonography. Gastroenterology. 2008;135:1100–1105.
Lieberman DA, Holub JL, Moravec MD, et al. Prevalence of colon polyps detected by colonoscopy screening in asymptomatic black and white patients. JAMA. 2008;300:1417–1422.
Brenner H, Hoffmeister M, Birkner B, et al. Men with negative results of guaiac-based fecal occult blood test have higher prevalence of colorectal neoplasms than women with positive results. Int J Cancer. 2014;134:2927–2934.
Ferlitsch M, Heinze G, Salzl P, et al. Sex is a stronger predictor of colorectal adenoma and advanced adenoma than fecal occult blood test. Med Oncol. 2014;31:151.
Inadomi JM, Vijan S, Janz NK, et al. Adherence to colorectal cancer screening: a randomized clinical trial of competing strategies. Arch Intern Med. 2012;172:575–582.
Auge J, Pellise M, Escudero JM, et al. Risk stratification for advanced colorectal neoplasia according to fecal hemoglobin concentration in a colorectal cancer screening program. Gastroenterology. 2014;147:628–636.
Van Hees F, Zauber AG, van Veldhuizen H, et al. The value of models in informing resource allocation in colorectal cancer screening—the case of the Netherlands. Gut. 2015;64:1985–1997.
Decker K, Demers A, Nugent Z, et al. Longitudinal rates of colon cancer screening use in Winnipeg, Canada: the experience of a universal health-care system with an organized colon screening program. Am J Gastroenterol. 2015;110:1640–1646.
Chan AT, Giovannucci EL. Primary prevention of colorectal cancer. Gastroenterology. 2010;138:2029–2043.
Acknowledgments
This project was supported with funding from NIDDK U01DK57132 and R33-DK61778-01. In addition, the practice network (Clinical Outcomes Research Initiative) has received support from the following entities to support the infrastructure of the practice-based network: AstraZeneca, Novartis, Bard International, Pentax USA, ProVation, Endosoft, GIVEN Imaging, and Ethicon. The commercial entities had no involvement in this research.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Dr. Lieberman is the executive director of CORI, a non-profit organization that receives funding from federal and industry sources. This potential conflict of interest has been reviewed and managed by the OHSU and Portland VA Conflict of Interest in Research Committees. All other authors have no potential conflicts of interests to disclose.
Rights and permissions
About this article
Cite this article
Mooers, H.M., Holub, J.L. & Lieberman, D.A. Screening Women Aged 50–59 for CRC Using Fecal Occult Blood Test Produces Outcomes Similar to Men Undergoing Screening Colonoscopy. Dig Dis Sci 63, 2780–2785 (2018). https://doi.org/10.1007/s10620-018-5156-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10620-018-5156-7