Opinion statement
Colorectal cancer (CRC) imposes significant morbidity and mortality, yet it is also largely preventable with evidence-based screening strategies. In May 2021, the US Preventive Services Task Force updated guidance, recommending screening begin at age 45 for average-risk individuals to reduce CRC incidence and mortality in the United States (US). The Task Force recommends screening with one of several screening strategies: high-sensitivity guaiac fecal occult blood test (HSgFOBT), fecal immunochemical test (FIT), multi-target stool DNA (mt-sDNA) test, computed tomographic (CT) colonography (virtual colonoscopy), flexible sigmoidoscopy, flexible sigmoidoscopy with FIT, or traditional colonoscopy. In addition to these recommended options, there are several emerging and novel CRC screening modalities that are not yet approved for first-line screening in average-risk individuals. These include blood-based screening or “liquid biopsy,” colon capsule endoscopy, urinary metabolomics, and stool-based microbiome testing for the detection of colorectal polyps and/or CRC. In order to maximize CRC screening uptake in the US, patients and providers should engage in informed decision-making about the benefits and limitations of recommended screening options to determine the most appropriate screening test. Factors to consider include the invasiveness of the test, test performance, screening interval, accessibility, and cost. In addition, health systems should have a programmatic approach to CRC screening, which may include evidence-based strategies such as patient education, provider education, mailed screening outreach, and/or patient navigation, to maximize screening participation.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Colorectal cancer (CRC) is the third most common cancer and the second leading cause of cancer-related deaths in the United States (US) [1••] . While there has been an overall decline in the number of CRC cases since the 1980s, incidence has been increasing in individuals under the age of 50 since the early 1990s [2]. Most CRCs develop through the adenoma-carcinoma sequence and can be identified with screening tests that aim to detect precancerous polyps (e.g., advanced adenomas), early cancers, or both [3••].
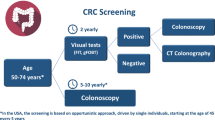
The US Preventive Services Task Force (USPSTF) recommends screening for all average-risk adults from age 45 to 49 (grade B recommendation) and from age 50 to 75 (grade A recommendation) by one of many screening strategies that vary on scientific approach, invasiveness, frequency of testing, cost, and availability [1, 4]. Modeling studies have shown that each of these screening modalities has the potential to increase life expectancy [5•]. Nonetheless, only 67.1% of adults age 50–75 were up-to-date with CRC screening in the US in 2019 [6], and screening rates vary by age, geographic region, insurance status, race/ethnicity, socioeconomic status, educational achievement, and source of healthcare [7]. There are also newer CRC screening modalities that are not yet recommended first line for average-risk individuals. In this piece, we summarize the test characteristics, advantages, disadvantages, and clinical practice considerations for currently available and emerging CRC screening modalities.
Screening modalities
The USPSTF currently recommends seven strategies for CRC screening. The stool-based CRC screening tests include the high-sensitivity guaiac fecal occult blood test (HSgFOBT), fecal immunochemical test (FIT), and multi-target stool DNA (mt-sDNA) test. The direct visualization screening tests include computed tomographic (CT) colonography, flexible sigmoidoscopy, and traditional colonoscopy. The seventh strategy is flexible sigmoidoscopy with a FIT, which combines a stool-based test and direct visualization [4••]. Emerging screening tests include colon capsule endoscopy (CCE), blood-based screening tests, stool-based microbiome studies, and urinary metabolomics.
Stool-based screening modalities
High-sensitivity guaiac-based fecal occult blood test
The HSgFOBT is a stool-based screening modality that aims to detect colorectal polyps and cancers through an oxidation reaction. When heme is present in a stool sample, the alpha-guaiaconic acid contained on the testing card is oxidized by the hydrogen peroxide reagent to create a blue color, representing an abnormal (positive) result [4, 8]. This screening strategy requires individuals to collect and submit three consecutive stool samples annually, and any one abnormal result warrants a colonoscopy to evaluate for colorectal polyps or cancers.
There is limited new data on HSgFOBT in the US in recent years due to heavy reliance on colonoscopy and other stool-based screening tests. The 2021 systematic review commissioned by the USPSTF reported a sensitivity for CRC of 0.50 to 0.75 (95% CI, 0.09–1.0) and specificity of 0.96–0.98 (95% CI, 0.95–0.99) (Hemoccult Sensa, Beckman Coulter) (Table 1) [1••]. Sensitivity for advanced adenomas is lower at 0.06–0.17 (95% CI, 0.02–0.23), while specificity for advanced adenomas is 0.96–0.99 (95% CI, 0.96–0.99) (Hemoccult Sensa, Beckman Coulter) [1••]. A 2019 meta-analysis of six trials over a 15-year period demonstrated that screening with gFOBT led to a reduction in CRC-related mortality (annual: RR 0.69; 95% CI, 0.56–0.86) but did not reduce CRC incidence (annual: RR 0.86; 95% CI, 0.72–1.03) [9•].
The benefits of this screening modality are that it is inexpensive, widely available, non-invasive, and can be performed outside of clinical settings. However, it does require dietary and medication restrictions. Many foods (i.e., red meat, rare meat, raw beets, carrots, cauliflower, cucumbers, grapefruit, mushrooms, broccoli, radish, horseradish, turnips) and medications (non-steriodal anti-inflammatory drugs, vitamin C, iron, vitamin E, blood thinners) must be avoided for 2 days prior to testing as they may cause an abnormal result. In addition, the test should not be performed in the presence of upper or lower gastrointestinal bleeding [8]. Although there are many different formulations of the FOBT, the 2021 USPSTF guidelines specifically recommend only newer high sensitivity formations (HSgFOBT) [1••].
Fecal Immunochemical Test
FIT is a stool-based screening test that uses an antibody assay to detect the presence of the intact globin portion of human hemoglobin in the stool [10]. There are numerous FIT products on the commercial market; however, the OC-Sensor test (Eiken Chemical) is commonly used due to its relatively high sensitivity and specificity [1••]. Based on the manufacturer’s recommended cut-off of 20μgHb/g feces, sensitivity and specificity for CRC are 0.74 (95% CI, 0.64–0.83) and 0.94 (95% CI, 0.93–0.96), respectively (Table 1) [1••]. Sensitivity and specificity for advanced adenoma are improved compared to HSgFOBT; sensitivity for advanced adenoma is 0.23 (95% CI, 0.20–0.25) and specificity is 0.96 (95% CI, 0.95–0.97) [1••]. A 2015 cohort study (n=5,417,699) demonstrated a reduction in CRC mortality with biennial FIT (RR 0.90, 95% CI, 0.84–0.95), but no change in CRC incidence [1••].
Unlike HSgFOBT, FIT requires only one stool sample annually and is not impacted by an individual’s diet or medications. In addition, it does not present an abnormal result in the presence of upper gastrointestinal bleeding as hemoglobin from foregut lesions is partially digested before it reaches the colon [10]. For these reasons and ease of use, it is currently the most common non-invasive CRC screening modality among average-risk individuals [7]. FIT is also highly accessible and less costly than screening colonoscopy and mt-sDNA [11]. In a 2020 comparative analysis, CRC detection rates were similar when four rounds of FIT every other year were compared to one-time flexible sigmoidoscopy and one-time colonoscopy [12•].
FIT does have its limitations, however. The 2021 USPSTF guidelines recommend that FIT be performed annually for CRC screening, which can require considerable healthcare resources for patient outreach [4••]. Like HSgFOBT and other non-colonoscopic screening modalities, it is a two-step screening strategy in which individuals with an abnormal result warrant a colonoscopy to complete the screening process [4••]. Colonoscopy completion rates after abnormal FIT are as low as 30% in some settings [13]. An additional focus is the appropriate cut-off value for an abnormal FIT in FIT-based population-based screening programs [3, 14,15,16]. Use of a low cut-off value (e.g.,10μgHgb/g) can increase sensitivity and positive predictive value but reduces specificity, requiring a larger number of follow-up colonoscopies and increasing the potential for adverse events during colonoscopy [14].
Multi-target stool DNA test
Cologuard (Exact Sciences) is a newer stool-based screening modality that aims to detect 11 molecular biomarkers for abnormal DNA (e.g., mutant KRAS, methylated BMP3, and methylated NDRG4), including human hemoglobin via a FIT [3••]. An abnormal test result is determined by a calculated score based on the presence and quantity of each biomarker [17]. It is the only mt-sDNA test currently commercially available [1••]. Its sensitivity and specificity for CRC are 0.93 (95% CI, 0.87–1.0) and 0.85 (95% CI, 0.84–0.86), respectively (Table 1) [1••]. For advanced adenoma, sensitivity is 0.43 (95% CI, 0.40–0.46) and specificity is 0.89 (95% CI, 0.86–0.92) [1••]. A 2021 study demonstrated that with perfect adherence, mt-sDNA reduces CRC incidence by 66% (95% CI, 63–68%) [11].
The benefits of mt-sDNA are that it can be performed in non-clinical settings, does not require diet or medication restrictions, can be performed every 3 years, and is non-invasive with low potential for adverse effects [4, 18]. Additionally, there may be increased sensitivity for right-sided colon lesions that are more often missed with other non-colonoscopic screening methods [19]. Exact Sciences also provides electronic and live patient navigation to guide patients through the screening process and increase test completion rates [17].
Challenges with mt-sDNA screening include cost, a high false positive rate compared to FIT, and some uncertainty as to whether further diagnostic work-up is necessary when the result is positive but the follow-up colonoscopy is negative [11, 20]. Currently, in this situation, the recommendation is that asymptomatic individuals should not undergo additional testing or procedures [3••].
Overall, when comparing mt-sDNA screening to FIT, mt-sDNA is better at differentiating between advanced precancerous lesions (advanced adenomas and serrated polyps ≥10mm and/or with low- or high-grade dysplasia) and non-neoplastic or negative findings (p=0.004) [21]. It also has a higher sensitivity for advanced adenomas, large and serrated lesions, multiple lesions, large lesions, and tubulovillous lesions compared to FIT [3••]. However, specificity is lower than FIT, which can result in more colonoscopies, more adverse events, and higher costs [3, 4]. There is ongoing research to improve the sensitivity and specificity of the mt-sDNA test, and as test characteristics and insurance coverage improve, use of this screening strategy will likely increase [22].
Direct visualization screening modalities
Computed tomographic colonography (virtual colonoscopy)
Virtual colonography uses a computed tomography (CT) scanner and computer reconstruction methods to visually evaluate the colon and rectum for colorectal polyps and cancers. For CRC screening, the recommended testing interval is 5 years, and individuals with abnormal findings must undergo traditional colonoscopy [23]. Sensitivity for adenomas 10mm or greater is 0.89 (95% CI, 0.83–0.96), and specificity is 0.94 (95% CI, 0.89–1.0) (Table 1) [1••]. For adenomas 6mm or larger, sensitivity is 0.86 (95% CI, 0.78–0.95), and specificity is 0.88 (95% CI, 0.83–0.95) [1••].
The benefits of CT colonography are that it is less invasive than colonoscopy, does not require sedation or anesthesia, has a low complication rate, and is relatively safe for individuals with medical comorbidities that preclude traditional colonoscopy [3, 24]. Compared to mt-sDNA, CT colonography detects more advanced adenomas and has a higher positive predictive value [18]. Unlike stool-based screening tests, it often allows for same-day endoscopic evaluation if needed [18]. Additionally, CT colonography can assess for invasive malignancy and the presence of metastasis [25] and has the potential to screen for other conditions, including poor bone mineral density, aortic calcification, and hepatic steatosis [18].
Potential unintended consequences of CT colonography include cumulative exposure of radiation with repeated examinations and the detection of incidental findings that require further work-up. The test results in clinically insignificant or indeterminate findings in 1.3–11.4% cases and potentially important findings in 3–4% of cases [1, 3, 4, 18]. Lack of colonoscopic follow-up after abnormal CT colonography is also a notable clinical challenge as with other forms of non-colonoscopic screening [23].
Flexible sigmoidoscopy
Flexible sigmoidoscopy is an endoscopic CRC screening modality that allows for direct visualization of the rectum, sigmoid colon, and descending colon. In addition, the procedure allows for resection or biopsy of distal colorectal lesions. Pooled data from four randomized control trials demonstrated a reduction in CRC incidence (IRR 0.78, 95% CI, 0.74–0.83) and CRC mortality (IRR 0.74, 95% CI, 0.68–0.80) with 1- or 2-time use of screening flexible sigmoidoscopy [26,27,28,29].
Benefits of flexible sigmoidoscopy over colonoscopy include reduced bleeding and perforation risk, avoidance of sedation and anesthesia, and lower cost [30, 31]. In addition, flexible sigmoidoscopy can be provided by a broader range of clinician specialists (e.g., primary care providers, family medicine providers).
The 2021 USPSTF guidelines recommend flexible sigmoidoscopy either alone every 5 years or every 10 years with an annual FIT [4••]. While flexible sigmoidoscopy every 5 years is associated with a similar number of life years gained as biennial FIT, the addition of FIT to flexible sigmoidoscopy results in a similar number of life years gained and similar mortality reduction to colonoscopy [5•]. The major limitations of flexible sigmoidoscopy are the inability to examine the entire colon (resulting in no incidence or mortality benefit for proximal CRCs) and low adherence [30]. Due to these limitations, flexible sigmoidoscopy (with or without FIT) is a less common strategy for CRC screening in the US and is largely reserved for clinical settings and populations with limited access to insurance, colonoscopy, and/or gastroenterologists.
Colonoscopy
Colonoscopy is the most common screening modality in the US and allows for visual examination of the entire colon and rectum for colorectal polyps and cancers. Sensitivity is 0.89–0.95 (95% CI, 0.70–0.99), and specificity is 0.89 (95% CI, 0.86–0.91) for adenomas 10mm or larger [1••]. For CRC, sensitivity is 0.18–1.0 (95% CI, 0.01–1.0) (Table 1) [1••].
In two large cohort studies, colonoscopy was associated with a reduction in CRC incidence (HR 0.53, 95% CI, 0.40–0.71) and a reduction in CRC-related mortality (HR 0.32, 95% CI, 0.24–0.45) [32, 33]. While studies consistently demonstrate a reduction in distal CRC incidence, the data are less consistent to support a reduction in incidence of proximal CRCs [30]. However, the overall reduction in CRC incidence and CRC-related mortality is greater for colonoscopy than for flexible sigmoidoscopy [30]. Additional benefits of colonoscopy are that it is a one-step definitive screening option, it is required only once every 10 years (if normal), and it allows for any detected lesions to be removed or biopsied.
Despite these benefits, colonoscopy requires bowel preparation and changes to diet and medication prior to the procedure. It is also seen by many patients as very invasive, causing patient hesitancy and non-adherence [1, 34, 35]. It is not available in some clinical settings, is more costly than the other recommended screening strategies, and is associated with a higher risk of complications [1, 11]. In a meta-analysis that included over 5 million colonoscopy procedures, serious adverse events included major bleeding (0.146%) and perforation (0.031%) [1••]. In addition, there is a high degree of inter-operator variability among gastroenterologists. An important and recent focus in CRC prevention and control has been to increase high-quality screening colonoscopy through the measurement, documentation, and improvement of several colonoscopy quality indicators that have been demonstrated to reduce development of interval CRCs [36,37,38].
Novel emerging screening modalities
Colon capsule endoscopy
CCE is an emerging screening tool that involves ingestion of a pill-sized wireless camera that takes images as it travels through the gastrointestinal tract [3••]. Colonoscopy is required for definitive screening if images reveal colorectal polyps or cancers [3••]. Though not currently recommended by the USPSTF or other medical professional societies as first-line screening for average-risk individuals, CCE is approved by the US Food and Drug Administration (FDA) for CRC screening in individuals with a history of incomplete colonoscopic evaluation or with high risk of complications during colonoscopy [3, 39]. The more recent CCE-2 (second generation) has a sensitivity of 76.7% (95% CI, 63.7–86.2%) and specificity of 90.7% (95% CI, 83.6–95.0%) for advanced neoplasia 10mm or larger (PillCam, Medtronic) (Table 1) [39]. For this screening modality, sensitivity is dependent on the percentage of colon surface area imaged and the time to capsule excretion, with a transit time of 3–5 h showing a sensitivity of 100% for advanced neoplasia greater than 6mm [39].
CCE is minimally invasive; does not require sedation, insufflation, or radiation; and can be completed in a non-clinical setting [3••]. It performs better than CT colonography in detecting neoplasia greater than 6mm in individuals with a history of incomplete colonoscopy and has lower risk for serious adverse events than traditional colonoscopy [39].
However, the complete CCE examination rate is only 66.7% [40], and 32% of CCEs lead to colonoscopy referral (polyp cutoff of 10mm or larger) [39]. CCE interpretation also requires a clinician that is trained in reading capsule endoscopy and often takes more time than performing a traditional colonoscopy and preparing a report [40]. There is ongoing development of more accurate and less expensive CCE devices that can image a larger surface area of the colon and rectum. [39]. Improvements in test performance and lower cost may make CCE more feasible and acceptable as a first-line screening method in the future.
Blood-based screening tests (liquid biopsy)
Blood-based cancer detection tests, also known as “liquid biopsy,” represent a new area of potential for single-cancer or multiple-cancer detection strategies. CRC develops through an accumulation of both genetic and epigenetic alterations in the gut mucosa, which serve as candidates for the development of blood-based screening tests [3••]. Currently, there is only one FDA-approved blood-based CRC screening test (Epi proColon; Epigenomics AG, 2016), and none are recommended as first-line screening in average-risk adults. Epi proColon detects circulating methylated SEPT9DNA and had a sensitivity of 0.68 (90% CI, 0.53–0.80) and specificity of 0.79 (95% CI, 0.77–0.81) for CRC in one nested case-control study (n=6,857) (Table 1) [41]. Sensitivity and specificity for advanced adenomas are 0.22 (95% CI, 0.18–0.24) and 0.79 (95% CI, 0.76–0.82), respectively [41]. The TriMeth test is another DNA methylation test and aims to detect three CRC-specific DNA methylation markers (C9orf50, KCNQ6, and CLIP4); it has a sensitivity of 0.80 and specificity of 0.99 for stage I CRCs in a recent discovery and validation study (CIs not provided) (Table 1) [42••].
Blood-based screening tests also include tests that detect plasma microRNA (miRNA) and plasma protein biomarkers. miRNA are expressed in the early phases of CRC development, are deregulated in precancerous and cancerous tissues, and remain relatively stable in the peripheral blood; therefore, they represent a unique area of interest in CRC screening [43]. In a 2020 analysis of 60 patients with an abnormal FIT result, the specificity of a combination of miRNAs was 0.19 for high grade adenomas and 0.26 for CRC when sensitivity was set at 0.85 (no CIs provided) (Table 1) [43]. Several plasma protein biomarkers (e.g., Interleukin-6, mannan-binding lectin serine protease 1, integrin alpha 11) are associated with CRC and are being actively evaluated in various combinations as potential screening strategies [44].
There is great interest in these tests given that they are minimally invasive and have the perceived potential for easy access and high patient adherence compared to the currently available screening methods [45]. However, factors yet to be determined include test accuracy, cost, and the appropriate follow-up after abnormal results. Additionally, the ability of these tests to detect precancerous polyps and CRC depends on the amount of tumor DNA shedding and the presence of circulating tumor cells and circulating tumor DNA in the peripheral blood. Unless high sensitivity is achieved with this technology, blood-based CRC screening can result in high numbers of false positive results, unnecessary colonoscopies, and adverse events during colonoscopy. In addition, the clinical implications of an abnormal blood-based screening test are uncertain when the follow-up colonoscopy is negative. It will be essential to determine the appropriate clinical work-up for other malignancies and conditions when colorectal polyps and CRC have been ruled-out. As with all innovation, blood-based screening tests must be evaluated in prospective, population-based studies to determine their accuracy at detecting colorectal polyps and cancers and their impact on CRC screening outcomes, including incidence, mortality, resource utilization, cost, and patient experience [46].
Stool-based microbiome tests
Stool-based microbiome tests are emerging screening modalities that are not yet FDA-approved or recommended for average-risk screening. The stool bacterial load can be higher in individuals with high-grade dysplasia and CRC, which has led to research to identify CRC bacterial markers in the stool [47]. However, given that these tests remain relatively new, there is limited data on test performance.
In a 2020 metagenomics and validation study, Lachnoclostridium sp. (m3), Fusobacterium nucleatum (Fn), and Clostridium hathewayi (Ch) were significantly enriched in adenoma [48]. At 78.5% specificity, fecal m3 had 48.3% sensitivity for adenoma and 62.1% sensitivity for CRC (Table 1). Fecal m3 performed better than Fn at identifying adenoma from controls (areas under the receiver operating characteristic curve (AUROCs) m3=0.675 vs Fn=0.620, p=0.09); however, Fn performed better at identifying CRC (AUROCs Fn=0.862 vs m3=0.741, p<0.0001). In a subgroup analysis in the same study, fecal m3 had higher sensitivity than FIT for non-advanced and advanced adenomas (44.2% and 50.8% for m3 vs. 0% and 16.1% for FIT). Overall, combining one or more fecal bacterial biomarkers with FIT optimized test performance; fecal m3, Fn, Ch, Bacteroides clarus, and FIT together had a sensitivity of 93.8% and specificity of 81.2% for CRC (no CIs provided) [48].
Like other stool-based screening modalities, stool-based microbiome tests will require follow-up colonoscopy when abnormal for definitive screening. An additional challenge with this screening strategy is that many current microbiome tests require genomic or metagenomic sequencing, which is time-consuming and expensive when compared to PCR tests [47]. Further research is needed to identify the optimal microbiome biomarkers and combinations of biomarkers and to evaluate test accuracy and cost.
Urine-based screening tests
Urine-based screening tests use liquid chromatography-mass spectrometry or nuclear magnetic resonance (NMR) spectroscopy to identify urine metabolites that are known to be associated with the presence of colorectal adenomas and CRC and represent another novel area in CRC screening [49, 50].
One study of a urine-based screening test that utilizes liquid chromatography-mass spectrometry found that diacetylspermine and kynurenine together in the urine had a specificity of 80.0% and sensitivity of 80.0% for CRC (no CIs provided) [49]. A study of a urine-based screening test that utilizes NMR spectroscopy identified a urine metabolomic profile with a sensitivity of 88.9% and specificity of 50.2% for adenomas [50]. Compared to gFOBT, this profile had a higher sensitivity for colorectal adenomas (size not specified) [50]. Finally, a recent study identified a urine metabolite profile that includes taurine, alanine, and 3-aminoisobutyrate and has a sensitivity of 75% and specificity of 100% for advanced adenomas and a sensitivity of 100% and specificity of 96.2% for CRC (no CIs provided) (Table 1) [51].
Some benefits of this screening modality are that it is non-invasive and samples can be collected in a non-clinical setting [49]. Additionally, NMR spectroscopy results are highly reproducible [51]. However, the current tests are limited by suboptimal sensitivity and specificity and their dependency on colonoscopy for definitive screening in the setting of an abnormal result [52]. Like most recently developed CRC screening modalities, there is no data on whether urine-based screening can impact CRC incidence and mortality, and they are not currently FDA-approved or formally recommended for CRC screening.
Promoting optimal screening strategies
Despite strong evidence that CRC screening improves CRC-related outcomes, screening rates in the US continue to be far below the National Colorectal Cancer Roundtable goal of 80% screened in every community [53]. There is evidence that providing patients with a choice between various screening modalities can increase screening participation [54, 55], and the USPSTF and other organizations have emphasized that “the best test is the one that gets done.” As such, it is essential for patients and providers to engage in an informed shared decision-making process to discuss the advantages and disadvantages of the available screening strategies and to assess a patient’s individual CRC risk and screening preferences (Table 2). In these patient-provider interactions, patients’ lack of knowledge about CRC risk, misinformation about CRC screening tests, mistrust in the healthcare system, and barriers to screening can often be addressed to help encourage screening utilization.
Beyond provider counseling, it is critical that health systems have organized strategies to encourage CRC screening. In the US, CRC screening is primarily opportunistic and only achieved if a primary care provider actively recommends it. Screening rates are higher in health systems that use evidence-based and programmatic approaches to screening, in which all patients who are eligible and due for screening receive automated interventions to maximize uptake. Some of the most effective evidence-based strategies include patient education [54•, 55•, 56•, 57•, 58•], provider education [54, 59], employing cadres of patient navigation [57, 60], and patient mailed outreach [54, 59,60,61]. Patient navigators help guide patients through the CRC screening process and the complexities of the health system, addressing educational, cultural, and logistical barriers that hinder screening completion and follow-up care while reducing burdens on primary care providers. Their use has been demonstrated to increase screening uptake by more than 100% [60]. Mailed outreach efforts include mailing FIT kits to patients due for screening, an approach that has also increased screening participation in population-based studies [13, 54, 57, 61]. Health systems with suboptimal CRC screening rates should be intentional in their population-health efforts and consider these population health strategies and others.
One of the main benefits of CRC screening is the broad range of available screening technologies, which offer great potential for both prevention and early detection. An understanding of the test characteristics, benefits, challenges, and clinical practice considerations for currently available and emerging CRC modalities is critical towards improving CRC screening uptake and CRC outcomes. In addition, further optimization of emerging screening technologies has the potential to decrease the overall burden of CRC in the US and globally.
References and Recommended Reading
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
•• Lin JS, Perdue LA, Henrikson NB, Bean SI, Blasi PR. Screening for colorectal cancer: updated evidence report and systematic review for the US Preventive Services Task Force. JAMA. 2021;325(19):1978–98. This evidence report systematically reviews the effectiveness, test accuracy, and harms of various colorectal cancer (CRC) screening tests. The review was used by the United States Preventive Services Task Force (USPSTF) to inform the 2021 USPSTF CRC screening recommendations. The authors provide quantitative data that allows healthcare providers to determine the best screening method for their patients.
Siegel RL, Fedewa SA, Anderson WF, Miller KD, Ma J, Rosenberg PS, et al. Colorectal cancer incidence patterns in the United States, 1974-2013. J Natl Cancer Inst. 2017;109(8):djw322.
•• Shaukat A, Kahi CJ, Burke CA, Rabeneck L, Sauer BG, Rex DK. ACG clinical guidelines: colorectal cancer screening 2021. Am J Gastroenterol. 2021;116(3):458–79. This article provides the American College of Gastroenterology’s updated colorectal cancer (CRC) screening guidelines. It discusses the efficacy of various screening modalities and outlines how screening recommendations differ for high-risk and average-risk individuals. Additionally, it discusses features of high-quality colonoscopy and different approaches for improving CRC screening adherence, including mailed fecal immunochemical test kits, patient navigation, and patient reminders.
•• Davidson KW, Barry MJ, Mangione CM, Cabana M, Caughey AB, Davis EM, et al. Screening for colorectal cancer: US Preventive Services Task Force Recommendation Statement. JAMA. 2021;325(19):1965–77. This systematic review was commissioned by the United States Preventive Services Task Force (USPSTF) to inform the 2021 colorectal cancer (CRC) screening recommendations. The USPSTF recommends that all average-risk adults age 45 to 49 (Grade B recommendation) and age 50 to 75 (Grade A recommendation) undergo screening; screening for individuals age 76 to 85 should be based on patient-provider discussions of harms and benefits. The recommendation to initiate screening at age 45 is based on modeling data for estimated life-years gained, CRC incidence, and CRC mortality if screening is started at varying ages. This manuscript also summarizes recommended screening modalities and intervals.
• Knudsen AB, Rutter CM, Peterse EFP, Lietz AP, Seguin CL, Meester RGS, et al. Colorectal Cancer Screening: An Updated Modeling Study for the US Preventive Services Task Force. JAMA. 2021;325(19):1998–2011. This publication is a comparative modeling study that uses three microsimulation models of colorectal cancer (CRC) screening in a hypothetical cohort to provide updated model-based estimates of the benefits and harms of CRC screening strategies and to identify strategies that may provide an efficient balance of life-years gained from screening and colonoscopy burden. The study was commissioned by the United States Preventive Services Task Force to inform the 2021 CRC screening recommendations.
Cancer Trends Progress Report Bethesda, MD: National Cancer Institute, NIH, DHHS; July 2021 [updated July 2, 2021. Available from: https://progressreport.cancer.gov.
Joseph DA, King JB, Dowling NF, Thomas CC, Richardson LC. Vital Signs: Colorectal Cancer Screening Test Use - United States, 2018. MMWR Morb Mortal Wkly Rep. 2020;69(10):253–9.
Kaur K, Adamski JJ. Fecal Occult Blood Test. StatPearls. Treasure Island (FL): StatPearls Publishing Copyright © 2021, StatPearls Publishing LLC; 2021.
• Jodal HC, Helsingen LM, Anderson JC, Lytvyn L, Vandvik PO, Emilsson L. Colorectal cancer screening with faecal testing, sigmoidoscopy or colonoscopy: a systematic review and network meta-analysis. BMJ Open. 2019;9(10):e032773. This study is a meta-analysis that aims to evaluate the effectiveness, benefits, and harms of fecal occult blood testing (gFOBT), sigmoidoscopy, and colonoscopy screening for colorectal cancer (CRC) over 15 years. Flexible sigmoidoscopy reduced CRC incidence, while annual gFOBT, biennial gFOBT, and sigmoidoscopy all reduced CRC mortality.
Wolf AMD, Fontham ETH, Church TR, Flowers CR, Guerra CE, LaMonte SJ, Etzioni R, McKenna M, Oeffinger KC, Shih YT, Walter LC, Andrews KS, Brawley OW, Brooks D, Fedewa SA, Manassaram-Baptiste D, Siegel RL, Wender RC, Smith RA. Colorectal cancer screening for average-risk adults: 2018 guideline update from the American Cancer Society. CA Cancer J Clin. 2018;68(4):250–81.
Redwood DG, Dinh TA, Kisiel JB, Borah BJ, Moriarty JP, Provost EM, Sacco FD, Tiesinga JJ, Ahlquist DA. Cost-effectiveness of multitarget stool DNA testing vs colonoscopy or fecal immunochemical testing for colorectal cancer screening in Alaska native people. Mayo Clin Proc. 2021;96(5):1203–17.
• Grobbee EJ, van der Vlugt M, van Vuuren AJ, Stroobants AK, Mallant-Hent RC, Lansdorp-Vogelaar I, et al. Diagnostic yield of one-time colonoscopy vs one-time flexible sigmoidoscopy vs multiple rounds of mailed fecal immunohistochemical tests in colorectal cancer screening. Clin Gastroenterol Hepatol. 2020;18(3):667–75.e1. This study compares the diagnostic yields of colonoscopy, flexible sigmoidoscopy, and fecal immunochemical tests (FITs) in colorectal cancer (CRC) screening. The authors found that FIT has a much higher participation rate and that multiple rounds of FIT detect more advanced neoplasia at the population level.
Green BB, Baldwin LM, West II, Schwartz M, Coronado GD. Low rates of colonoscopy follow-up after a positive fecal immunochemical test in a Medicaid health plan delivered mailed colorectal cancer screening program. J Prim Care Community Health. 2020;11:2150132720958525.
Njor SH, Andersen B, Friis-Hansen L, de Haas N, Linnemann D, Nørgaard H, et al. The optimal cut-off value in fit-based colorectal cancer screening: an observational study. Cancer Med. 2021;10(5):1872–9.
Sarkeala T, Färkkilä M, Anttila A, Hyöty M, Kairaluoma M, Rautio T, Voutilainen M, Helander S, Jäntti M, Lehtinen M, Patrikka L, Malila N, Heinävaara S. Piloting gender-oriented colorectal cancer screening with a faecal immunochemical test: population-based registry study from Finland. BMJ Open. 2021;11(2):e046667.
Clark GRC, Strachan JA, McPherson A, Digby J, Mowat C, Steele RJC, Fraser CG. Faecal haemoglobin distributions by sex, age, deprivation and geographical region: consequences for colorectal cancer screening strategies. Clin Chem Lab Med. 2020;58(12):2073–80.
Weiser E, Parks PD, Swartz RK, Thomme JV, Lavin PT, Limburg P, Berger BM. Cross-sectional adherence with the multi-target stool DNA test for colorectal cancer screening: real-world data from a large cohort of older adults. J Med Screen. 2021;28(1):18–24.
Pickhardt PJ, Graffy PM, Weigman B, Deiss-Yehiely N, Hassan C, Weiss JM. Diagnostic performance of multitarget stool DNA and CT colonography for noninvasive colorectal cancer screening. Radiology. 2020;297(1):120–9.
Eckmann JD, Ebner DW, Bering J, Kahn A, Rodriguez E, Devens ME, Lowrie KL, Doering K, Then S, Burger KN, Mahoney DW, Prichard DO, Wallace MB, Gurudu SR, Finney LJ, Limburg P, Berger B, Ahlquist DA, Kisiel JB. Multitarget stool DNA screening in clinical practice: high positive predictive value for colorectal neoplasia regardless of exposure to previous colonoscopy. Am J Gastroenterol. 2020;115(4):608–15.
Imperiale TF, Ransohoff DF, Itzkowitz SH, Levin TR, Lavin P, Lidgard GP, Ahlquist DA, Berger BM. Multitarget stool DNA testing for colorectal-cancer screening. N Engl J Med. 2014;370(14):1287–97.
Bosch LJW, Melotte V, Mongera S, Daenen KLJ, Coupé VMH, van Turenhout ST, Stoop EM, de Wijkerslooth TR, Mulder CJJ, Rausch C, Kuipers EJ, Dekker E, Domanico MJ, Lidgard GP, Berger BM, van Engeland M, Carvalho B, Meijer GA. Multitarget stool DNA test performance in an average-risk colorectal cancer screening population. Am J Gastroenterol. 2019;114(12):1909–18.
Carethers JM. Fecal DNA testing for colorectal cancer screening. Annu Rev Med. 2020;71:59–69.
Lynn W, Vadhwana B, Bell DJ, Borgstein R, Demetriou G, Nair MS, Meleagros L. Computed tomography colonography: a retrospective analysis of outcomes of 2 years experience in a district general hospital. ANZ J Surg. 2019;89(5):541–5.
Sha J, Chen J, Lv X, Liu S, Chen R, Zhang Z. Computed tomography colonography versus colonoscopy for detection of colorectal cancer: a diagnostic performance study. BMC Med Imaging. 2020;20(1):51.
Tang WJ, Nie Z, Fan WL, Cheng L, Lei ZQ, Yang M. Diagnostic Value of 128-slice Spiral CT combined with virtual colonoscopy for colorectal cancer. Curr Med Sci. 2019;39(1):146–52.
Holme Ø, Løberg M, Kalager M, Bretthauer M, Hernán MA, Aas E, Eide TJ, Skovlund E, Lekven J, Schneede J, Tveit KM, Vatn M, Ursin G, Hoff G, NORCCAP Study Group†. Long-term effectiveness of sigmoidoscopy screening on colorectal cancer incidence and mortality in women and men: a randomized trial. Ann Intern Med. 2018;168(11):775–82.
Miller EA, Pinsky PF, Schoen RE, Prorok PC, Church TR. Effect of flexible sigmoidoscopy screening on colorectal cancer incidence and mortality: long-term follow-up of the randomised US PLCO cancer screening trial. Lancet Gastroenterol Hepatol. 2019;4(2):101–10.
Atkin W, Wooldrage K, Parkin DM, Kralj-Hans I, MacRae E, Shah U, Duffy S, Cross AJ. Long term effects of once-only flexible sigmoidoscopy screening after 17 years of follow-up: the UK flexible sigmoidoscopy screening randomised controlled trial. Lancet. 2017;389(10076):1299–311.
Segnan N, Armaroli P, Bonelli L, Risio M, Sciallero S, Zappa M, Andreoni B, Arrigoni A, Bisanti L, Casella C, Crosta C, Falcini F, Ferrero F, Giacomin A, Giuliani O, Santarelli A, Visioli CB, Zanetti R, Atkin WS, et al. Once-only sigmoidoscopy in colorectal cancer screening: follow-up findings of the Italian Randomized Controlled Trial--SCORE. J Natl Cancer Inst. 2011;103(17):1310–22.
Ko CW, Doria-Rose VP, Barrett MJ, Kamineni A, Enewold L, Weiss NS. Screening colonoscopy and flexible sigmoidoscopy for reduction of colorectal cancer incidence: A case-control study. PLoS One. 2019;14(12):e0226027.
Ko CW, Doria-Rose VP, Barrett MJ, Kamineni A, Enewold L, Weiss NS. Screening flexible sigmoidoscopy versus colonoscopy for reduction of colorectal cancer mortality. Int J Colorectal Dis. 2019;34(7):1273–81.
García-Albéniz X, Hsu J, Bretthauer M, Hernán MA. Effectiveness of screening colonoscopy to prevent colorectal cancer among Medicare beneficiaries aged 70 to 79 years: a prospective observational study. Ann Intern Med. 2017;166(1):18–26.
Nishihara R, Wu K, Lochhead P, Morikawa T, Liao X, Qian ZR, Inamura K, Kim SA, Kuchiba A, Yamauchi M, Imamura Y, Willett WC, Rosner BA, Fuchs CS, Giovannucci E, Ogino S, Chan AT. Long-term colorectal-cancer incidence and mortality after lower endoscopy. N Engl J Med. 2013;369(12):1095–105.
Denberg TD, Melhado TV, Coombes JM, Beaty BL, Berman K, Byers TE, Marcus AC, Steiner JF, Ahnen DJ. Predictors of nonadherence to screening colonoscopy. J Gen Intern Med. 2005;20(11):989–95.
Brown LJ, Roeger SL, Reed RL. Patient perspectives on colorectal cancer screening and the role of general practice. BMC Fam Pract. 2019;20(1):109.
Rex DK, Schoenfeld PS, Cohen J, Pike IM, Adler DG, Fennerty MB, et al. Quality indicators for colonoscopy. Am J Gastroenterol. 2015;110(1):72–90.
May FP, Shaukat A. State of the science on quality indicators for colonoscopy and how to achieve them. Am J Gastroenterol. 2020;115(8):1183–90.
•• Lund M, Trads M, Njor SH, Erichsen R, Andersen B. Quality indicators for screening colonoscopy and colonoscopist performance and the subsequent risk of interval colorectal cancer: a systematic review. JBI Database System Rev Implement Rep. 2019;17(11):2265–300. This publication examines the relationship between several colonoscopy quality indicators and interval colorectal cancers (CRC). Quality indicators discussed include withdrawal time, cecal intubation rate, and adenoma detection rate. The authors recommend several benchmarks for screening colonoscopy to minimize risk of interval CRC: withdrawal time greater than 6 minutes, cecal intubation rate greater than or equal to 90%, and adenoma detection rate greater than or equal to 25%.
Pecere S, Senore C, Hassan C, Riggi E, Segnan N, Pennazio M, et al. Accuracy of colon capsule endoscopy for advanced neoplasia. Gastrointest Endosc. 2020;91(2) 406-14.e1
Kroijer R, Kobaek-Larsen M, Qvist N, Knudsen T, Baatrup G. Colon capsule endoscopy for colonic surveillance. Colorectal Dis. 2019;21(5):532–7.
Potter NT, Hurban P, White MN, Whitlock KD, Lofton-Day CE, Tetzner R, Koenig T, Quigley NB, Weiss G. Validation of a real-time PCR-based qualitative assay for the detection of methylated SEPT9 DNA in human plasma. Clin Chem. 2014;60(9):1183–91.
•• Jensen S, Øgaard N, Ørntoft MW, Rasmussen MH, Bramsen JB, Kristensen H, et al. Novel DNA methylation biomarkers show high sensitivity and specificity for blood-based detection of colorectal cancer-a clinical biomarker discovery and validation study. Clin Epigenetics. 2019;11(1):158. This study is a discovery and validation study that aimed to identify and characterize DNA methylation biomarkers for colorectal cancer (CRC). The authors found that TriMeth (includes three CRC-specific DNA methylation markers) has a sensitivity of 0.80 and specificity of 0.99 for stage I CRC.
Zanutto S, Ciniselli CM, Belfiore A, Lecchi M, Masci E, Delconte G, Primignani M, Tosetti G, Dal Fante M, Fazzini L, Airoldi A, Vangeli M, Turpini F, Rubis Passoni GG, Viaggi P, Arena M, Motta RIO, Cantù AM, Crosta C, et al. Plasma miRNA-based signatures in CRC screening programs. Int J Cancer. 2020;146(4):1164–73.
Bhardwaj M, Weigl K, Tikk K, Benner A, Schrotz-King P, Brenner H. Multiplex screening of 275 plasma protein biomarkers to identify a signature for early detection of colorectal cancer. Mol Oncol. 2020;14(1):8–21.
Ferrari A, Neefs I, Hoeck S, Peeters M, Van Hal G. Towards novel non-invasive colorectal cancer screening methods: a comprehensive review. Cancers (Basel). 2021;13(8):1820.
Beer TM. Examining developments in multicancer early detection: highlights of new clinical data from recent conferences. Am J Manag Care. 2021;27(19 Suppl):S347–s55.
Grobbee EJ, Lam SY, Fuhler GM, Blakaj B, Konstantinov SR, Bruno MJ, Peppelenbosch MP, Kuipers EJ, Spaander MCW. First steps towards combining faecal immunochemical testing with the gut microbiome in colorectal cancer screening. United European Gastroenterol J. 2020;8(3):293–302.
Liang JQ, Li T, Nakatsu G, Chen YX, Yau TO, Chu E, et al. A novel faecal Lachnoclostridium marker for the non-invasive diagnosis of colorectal adenoma and cancer. Gut. 2020;69(7):1248–57.
Deng L, Ismond K, Liu Z, Constable J, Wang H, Alatise OI, Weiser MR, Kingham TP, Chang D. Urinary Metabolomics to identify a unique biomarker panel for detecting colorectal cancer: a multicenter study. Cancer Epidemiol Biomarkers Prev. 2019;28(8):1283–91.
Wang H, Tso V, Wong C, Sadowski D, Fedorak RN. Development and validation of a highly sensitive urine-based test to identify patients with colonic adenomatous polyps. Clin Transl Gastroenterol. 2014;5(3):e54.
Kim ER, Kwon HN, Nam H, Kim JJ, Park S, Kim YH. Urine-NMR metabolomics for screening of advanced colorectal adenoma and early stage colorectal cancer. Sci Rep. 2019;9(1):4786.
Barichello S, Deng L, Ismond KP, Loomes DE, Kirwin EM, Wang H, Chang D, Svenson LW, Thanh NX. Comparative effectiveness and cost-effectiveness analysis of a urine metabolomics test vs. alternative colorectal cancer screening strategies. Int J Colorectal Dis. 2019;34(11):1953–62.
Wender RC, Doroshenk M, Brooks D, Hotz J, Smith RA. Creating and implementing a national public health campaign: the American Cancer Society's and National Colorectal Cancer Roundtable's 80% by 2018 initiative. Am J Gastroenterol. 2018;113(12):1739–41.
• Inadomi JM, Issaka RB, Green BB. What multilevel interventions do we need to increase the colorectal cancer screening rate to 80%? Clin Gastroenterol Hepatol. 2021;19(4):633–45. This paper examines the effectiveness of various interventions to increase colorectal cancer screening uptake. The most effective interventions to increase screening are mailed outreach and patient navigation. This paper also purports the importance of multi-level and multi-component interventions to increase screening, targeting several levels of the cancer screening continuum from the patient, provider/health delivery teams, family and social support, and the healthcare environment.
Inadomi JM, Vijan S, Janz NK, Fagerlin A, Thomas JP, Lin YV, et al. Adherence to colorectal cancer screening: a randomized clinical trial of competing strategies. Archives of Internal Medicine. 2012;172(7):575–82.
Gupta S, Halm EA, Rockey DC, Hammons M, Koch M, Carter E, Valdez L, Tong L, Ahn C, Kashner M, Argenbright K, Tiro J, Geng Z, Pruitt S, Skinner CS. Comparative effectiveness of fecal immunochemical test outreach, colonoscopy outreach, and usual care for boosting colorectal cancer screening among the underserved: a randomized clinical trial. JAMA Intern Med. 2013;173(18):1725–32.
Singal AG, Gupta S, Tiro JA, Skinner CS, McCallister K, Sanders JM, Bishop WP, Agrawal D, Mayorga CA, Ahn C, Loewen AC, Santini NO, Halm EA. Outreach invitations for FIT and colonoscopy improve colorectal cancer screening rates: A randomized controlled trial in a safety-net health system. Cancer. 2016;122(3):456–63.
Baker DW, Brown T, Buchanan DR, Weil J, Balsley K, Ranalli L, Lee JY, Cameron KA, Ferreira MR, Stephens Q, Goldman SN, Rademaker A, Wolf MS. Comparative effectiveness of a multifaceted intervention to improve adherence to annual colorectal cancer screening in community health centers: a randomized clinical trial. JAMA Intern Med. 2014;174(8):1235–41.
Yen T, Qin F, Sundaram V, Asiimwe E, Storage T, Ladabaum U. Randomized controlled trial of personalized colorectal cancer risk assessment vs education to promote screening uptake. Am J Gastroenterol. 2021;116(2):391–400.
Dougherty MK, Brenner AT, Crockett SD, Gupta S, Wheeler SB, Coker-Schwimmer M, Cubillos L, Malo T, Reuland DS. Evaluation of interventions intended to increase colorectal cancer screening rates in the United States: A Systematic Review and Meta-analysis. JAMA Intern Med. 2018;178(12):1645–58.
Somsouk M, Rachocki C, Mannalithara A, Garcia D, Laleau V, Grimes B, Issaka RB, Chen E, Vittinghoff E, Shapiro JA, Ladabaum U. Effectiveness and cost of organized outreach for colorectal cancer screening: a randomized, controlled trial. J Natl Cancer Inst. 2020;112(3):305–13.
Bretagne JF, Piette C, Cosson M, Durand G, Lièvre A. Switching from guaiac to immunochemical faecal occult blood test increases participation and diagnostic yield of colorectal cancer screening. Dig Liver Dis. 2019;51(10):1461–9.
Deding U, Bjørsum-Meyer T, Kaalby L, Kobaek-Larsen M, Thygesen MK, Madsen JB, Kroijer R, Baatrup G. Colon capsule endoscopy in colorectal cancer screening: Interim analyses of randomized controlled trial CareForColon2015. Endosc Int Open. 2021;9(11):E1712–e9.
Berry E, Miller S, Koch M, Balasubramanian B, Argenbright K, Gupta S. Lower abnormal fecal immunochemical test cut-off values improve detection of colorectal cancer in system-level screens. Clin Gastroenterol Hepatol. 2020;18(3):647–53.
Randel KR, Schult AL, Botteri E, Hoff G, Bretthauer M, Ursin G, et al. Colorectal cancer screening with repeated fecal immunochemical test versus sigmoidoscopy: baseline results from a randomized trial. Gastroenterology. 2021;160(4) 1085-96.e5
Peterse EFP, Meester RGS, de Jonge L, Omidvari AH, Alarid-Escudero F, Knudsen AB, Zauber AG, Lansdorp-Vogelaar I. Comparing the cost-effectiveness of innovative colorectal cancer screening tests. J Natl Cancer Inst. 2021;113(2):154–61.
Regge D, Iussich G, Segnan N, Correale L, Hassan C, Arrigoni A, Asnaghi R, Bestagini P, Bulighin G, Cassinis MC, Ederle A, Ferraris A, Galatola G, Gallo T, Gandini G, Garretti L, Martina MC, Molinar D, Montemezzi S, et al. Comparing CT colonography and flexible sigmoidoscopy: a randomised trial within a population-based screening programme. Gut. 2017;66(8):1434–40.
Stoop EM, de Haan MC, de Wijkerslooth TR, Bossuyt PM, van Ballegooijen M, Nio CY, van de Vijver MJ, Biermann K, Thomeer M, van Leerdam ME, Fockens P, Stoker J, Kuipers EJ, Dekker E. Participation and yield of colonoscopy versus non-cathartic CT colonography in population-based screening for colorectal cancer: a randomised controlled trial. Lancet Oncol. 2012;13(1):55–64.
Sawhney TG, Pyenson BS, Rotter D, Berrios M, Yee J. Computed tomography colonography less costly than colonoscopy for colorectal cancer screening of commercially insured patients. Am Health Drug Benefits. 2018;11(7):353–61.
Castells A, Bessa X, Quintero E, Bujanda L, Cubiella J, Salas D, Lanas A, Carballo F, Morillas JD, Hernández C, Jover R, Montalvo I, Arenas J, Cosme A, Hernández V, Iglesias B, Castro I, Cid L, Sala T, et al. Risk of advanced proximal neoplasms according to distal colorectal findings: comparison of sigmoidoscopy-based strategies. J Natl Cancer Inst. 2013;105(12):878–86.
Adler A, Geiger S, Keil A, Bias H, Schatz P, de Vos T, et al. Improving compliance to colorectal cancer screening using blood and stool based tests in patients refusing screening colonoscopy in Germany. BMC Gastroenterol. 2014;14:183.
Roth JA, de Vos T, Ramsey SD. Clinical and budget impact of increasing colorectal cancer screening by blood- and stool-based testing. Am Health Drug Benefits. 2019;12(5):256–62.
Piscitello A, Saoud L, Fendrick AM, Borah BJ, Hassmiller Lich K, Matney M, Ozbay AB, Parton M, Limburg PJ. Estimating the impact of differential adherence on the comparative effectiveness of stool-based colorectal cancer screening using the CRC-AIM microsimulation model. PLoS One. 2020;15(12):e0244431.
Pilonis ND, Bugajski M, Wieszczy P, Rupinski M, Pisera M, Pawlak E, Regula J, Kaminski MF. Participation in competing strategies for colorectal cancer screening: a randomized health services study (PICCOLINO Study). Gastroenterology. 2021;160(4):1097–105.
Funding
FPM is supported by the UCLA Jonsson Comprehensive Cancer Center and the Eli and Edythe Broad Center of Regenerative Medicine and Stem Cell Research Ablon Scholars Program.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
Shailavi Jain declares that she has no conflict of interest. Jetrina Maque declares that she has no conflict of interest. Artin Galoosian declares that he has no conflict of interest. Antonia Osuna-Garcia declares that she has no conflict of interest. Folasade P. May receives research funding from Exact Sciences and has received compensation for service as a consultant from Freenome, Medtronic, and Takeda.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article is part of the Topical Collection on Lower Gastrointestinal Cancers
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Jain, S., Maque, J., Galoosian, A. et al. Optimal Strategies for Colorectal Cancer Screening. Curr. Treat. Options in Oncol. 23, 474–493 (2022). https://doi.org/10.1007/s11864-022-00962-4
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11864-022-00962-4