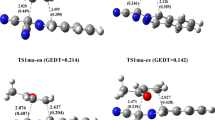
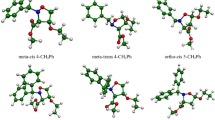
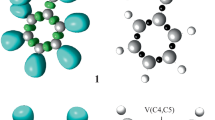
The selectivity and the molecular mechanism of the intramolecular [3+2] cycloaddition reaction of a nitrone–vinylphosphonate adduct was computationally studied within the molecular electron density theory using density functional theory method at the B3LYP/6-31G(d,p) level of theory. Conceptual density functional theory indices show that the nitrone–vinylphosphonate adduct has dual strong electrophilic and nucleophilic character. Local Parr functions reactivity indices reveal that this reaction favors the formation of the fused regioisomers in accordance with the experimental data. Analysis of different energetic profiles indicates that the fused-endo competitive pathway is favored kinetically, whereby this intramolecular reaction is characterized by exothermic and exergonic character. The geometry of transition states structures shows that the mechanism of this cycloaddition reaction is synchronous. Electron localization function topological analysis of the changes in electron density during the most favored reaction pathway shows that the mechanism is synchronous non-concerted.








Similar content being viewed by others
References
Kaur, N. J. Heterocycl. Chem. 2015, 52, 953.
Carruthers, W. Cycloaddition Reactions in Organic Synthesis; Pergamon Press: London, 1990. eBook; Elsevier, 2013.
Huisgen, R. In 1,3-Dipolar Cycloaddition Chemistry; Padwa, A., Ed.; Wiley: New York, 1984, Vol. 1, p. 55.
Gothelf, K. V.; Jørgensen, K. A. Chem. Rev. 1998, 98, 863.
Berthet, M.; Cheviet, T.; Dujardin, G.; Parrot, I.; Martinez, J. Chem. Rev. 2016, 116, 15235.
Sadashiva, M. P.; Mallesha, H.; Murthy, K. K.; Rangappa, K. S. Bioorg. Med. Chem. Lett. 2005, 15, 1811.
Żelechowski, K.; Gołębiewski, W. M.; Krawczyk, M. Monatsh. Chem. 2015, 146, 1895.
Mullen, G. B.; Swift, P. A.; Georgiev, V. S. J. Pharm. Sci. 1987, 76, 930.
Kaur, M.; Singh, B.; Singh, B.; Arjuna, A. J. Heterocycl. Chem. 2017, 54, 1348.
Groaz, E.; De Jonghe, S. Front. Chem. 2020, 8, 616863.
Sennikova, V. V; Zalaltdinova, A. V; Sadykova, Y. M.; Khamatgalimov, A. R.; Gazizov, A. S.; Voloshina, A. D.; Lyubina, A. P.; Amerhanova, S. K.; Voronina, J. K.; Chugunova, E. A.; Appazov, N. O.; Burilov, A. R.; Pudovik, M. A. Int. J. Mol. Sci. 2022, 23, 14348.
Turhanen, P. A. J. Biomed. Res. Environ. Sci. 2022, 3, 195.
Duret, G.; Quinlan, R.; Yin, B.; Martin, R. E.; Bisseret, P.; Neuburger, M.; Gandon, V.; Blanchard, N. J. Org. Chem. 2017, 82, 1726.
Chafaa, F.; Hellel, D.; Khorief, A.; Djerourou, A. A. Tetrahedron Lett. 2016, 57, 67.
Chafaa, F.; Nacereddine, A. K.; Djerourou, A. Lett. Org. Chem. 2020, 17, 260.
Domingo, L. R.; Ríos-Gutiérrez, M.; Adjieufack, A. I.; Ndassa, I. M.; Nouhou, C. N.; Mbadcam, J. K. ChemistrySelect 2018, 3, 5412.
Jasiński, R.; Dresler, E. Organics 2020, 1, 49.
Jasiński, R. Tetrahedron 2013, 69, 927.
Sobhi, C.; Khorief Nacereddine, A.; Djerourou, A.; Ríos-Gutiérrez, M.; Domingo, L. R. J. Phys. Org. Chem. 2017, 30, e3637.
Żmigrodzka, M.; Sadowski, M.; Kras, J.; Dresler, E.; Demchuk, O. M.; Kula, K. Sci. Rad. 2022, 1, 26.
Barama, L.; Bayoud, B.; Chafaa, F.; Nacereddine, A. K.; Djerourou, A. Struct. Chem. 2018, 29, 1709.
Lamri, S.; Heddam, A.; Kara, M.; Yahia, W.; Khorief Nacereddine, A. Organics 2021, 2, 57.
Hamdaoui, H.; Khorief Nacereddine, A.; Djerourou, A. J. Phys. Org. Chem. 2022, 36, e4462.
Sadi, S.; Khorief Nacereddine, A.; Djerourou, A. J. Phys. Org. Chem. 2022, 35, e4311.
Domingo, L. R. Molecules 2016, 21, 1319.
Ríos-Gutiérrez, M.; Domingo, L. R. Eur. J. Org. Chem. 2019, 267.
Koumbis, A. E.; Gallos, J. K. Curr. Org. Chem. 2003, 7, 585.
Tufariello, J. J. In 1,3-Dipolar Cycloaddition Chemistry; Padwa, A., Ed.; Wiley: New York, 1984, vol. 2, p. 83.
Huang, T.; Wang, Q.; Kong, D.; Wu, M. Tetrahedron Lett. 2019, 60, 150913.
Domingo, L. R.; Aurell, M. J.; Pérez, P.; Contreras, R. Tetrahedron 2002, 58, 4417.
Jaramillo, P.; Domingo, L. R.; Chamorro, E.; Pérez, P. A J. Mol. Struct.: THEOCHEM 2008, 865, 68.
Domingo, L. R.; Sáez, J. A. Org. Biomol. Chem. 2009, 7, 3576.
Domingo, L. R.; Pérez, P.; Sáez, J. A. RSC Adv. 2013, 3, 1486.
Domingo, L. R. RSC Adv. 2014, 4, 32415.
Emamian, S.; Lu, T.; Domingo, L. R.; Heidarpoor Saremi, L.; Ríos-Gutiérrez, M. Chem. Phys. 2018, 501, 128.
Benchouk, W.; Mekelleche, S. M.; Silvi, B.; Aurell, M. J.; Domingo, L. R. J. Phys. Org. Chem. 2011, 24, 611.
Becke, A. D.; Edgecombe, K. E. J. Chem. Phys. 1990, 92, 5397.
Nacereddine, A. K. J. Mol. Model. 2020, 26, 328.
Yahia, W.; Nacereddine, A. K.; Djerourou, A. Intl. J. Quantum Chem. 2017, 118, e25540.
Chafaa, F.; Nacereddine, A. K.; Djerourou, A. Theor. Chem. Acc. 2019, 123.
Domingo, L. R.; Sáez, J. A. J. Org. Chem. 2011, 76, 373.
Frisch, M. J.; Trucks, G. W.; Schlegel, H. B.; Scuseria, G. E.; Robb, Ma.; Cheeseman, J. R.; Scalmani, G.; Barone, V.; Mennucci, B.; Petersson, G. A.; Nakatsuji, H.; Li, X.; Caricato, M.; Marenich, A.; Bloino, J.; Janesko, B. G.; Gomperts, R.; Mennucci, B.; Hratchian, H. P.; Ortiz, J. V.; Izmaylov, A. F.; Sonnenberg, J. L.; Williams-Young, D.; Ding, F.; Lipparini, F.; Egidi, F.; Goings, J.; Peng, B.; Petrone, A.; Henderson, T.; Ranasinghe, D.; Zakrzewski, V. G.; Gao, J.; Rega, N.; Zheng, G.; Liang, W.; Hada, M.; Ehara, M.; Toyota, K.; Fukuda, R.; Hasegawa, J.; Ishida, M.; Nakajima, T.; Honda, Y.; Kitao, O.; Nakai, H.; Vreven, T.; Throssell, K.; Montgomery, J. A., Jr.; Peralta, J. E.; Ogliaro, F.; Bearpark, M.; Heyd, J. J.; Brothers, E.; Kudin, K. N.; Staroverov, V. N.; Keith, T.; Kobayashi, R.; Normand, J.; Raghavachari, K.; Rendell, A.; Burant, J. C.; Iyengar, S. S.; Tomasi, J.; Cossi, M.; Millam, J. M.; Klene, M.; Adamo, C.; Cammi, R.; Ochterski, J. W.; Martin, R. L.; Morokuma, K.; Farkas, O.; Foresman, J. B.; Fox, D. J. Gaussian 09, Revision A.02; Gaussian, Inc.: Wallingford, 2016.
Lee, C.; Yang, W.; Parr, R. G. Phys. Rev. B: Condens. Matter Mater. Phys. 1988, 37, 785.
Becke, A. D. Phys. Rev. A: At., Mol., Opt. Phys. 1988, 38, 3098.
Ríos-Gutiérrez, M.; Domingo, L. R.; Jasiński, R. RSC Adv. 2021, 11, 9698.
Mitka, K.; Fela, K.; Olszewska, A.; Jasiński, R. Molecules 2021, 26, 7147.
Layeb, H.; Nacereddine, A. K.; Djerourou, A.; Ríos-Gutiérrez, M.; Domingo, L. R. J. Mol. Model. 2015, 21, 104.
Tomasi, J.; Persico, M. Chem. Rev. 1994, 94, 2027.
Cancès, E.; Mennucci, B.; Tomasi, J. J. Chem. Phys. 1997, 107, 3032.
Cossi, M.; Barone, V.; Cammi, R.; Tomasi, J. Chem. Phys. Lett. 1996, 255, 327.
Becke, A. D. J. Chem. Phys. 1993, 98(7), 5648.
Reed, A. E.; Weinstock, R. B.; Weinhold, F. J. Chem. Phys. 1985, 83, 735.
Reed, A. E.; Curtiss, L. A.; Weinhold, F. Chem. Rev. 1988, 88, 899.
Fukui, K. J. Phys. Chem. 1970, 74, 4161.
Gonzalez, C.; Schlegel, H. B. J. Chem. Phys. 1991, 95, 5853.
Geerlings, P.; De Proft, F.; Langenaeker, W. Chem. Rev. 2003, 103, 1793.
Domingo, L. R.; Ríos-Gutiérrez, M.; Pérez, P. Molecules 2016, 21, 748.
Parr, R. G.; Pearson, R. G. J. Am. Chem. Soc. 1983, 105, 7512.
Parr, R. G.; Szentpály, L. v.; Liu, S. J. Am. Chem. Soc. 1999, 12, 1922.
Domingo, L. R.; Chamorro, E.; Pérez, P. J. Org. Chem. 2008, 73, 4615.
Lu, T.; Chen, F. J. Comput. Chem. 2012, 33, 580.
This work was supported by the Ministry of Higher Education and Scientific Research of the Algerian Government (project PRFU Code: B00L01EN210120220001).
Author information
Authors and Affiliations
Corresponding author
Additional information
Published in Khimiya Geterotsiklicheskikh Soedinenii, 2023, 59(3), 171–178
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Chafaa, F., Nacereddine, A.K. A molecular electron density theory study of mechanism and selectivity of the intramolecular [3+2] cycloaddition reaction of a nitrone–vinylphosphonate adduct. Chem Heterocycl Comp 59, 171–178 (2023). https://doi.org/10.1007/s10593-023-03179-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10593-023-03179-x