Abstract
We evaluated the effect of the Great Tohoku earthquake, which occurred on March 11, 2011 in Japan, on the genetic diversity and population structure of Pacific herring (Clupea pallasii). Pacific herring (n = 4466) were collected between 2003 and 2014 through more than 20 sampling events during spawning periods at nine spawning sites throughout the Pacific herring distribution range in Japan. We measured them and genotyped 3784 fish at five microsatellite loci. Following the tsunami, the sea-spawning population at the center of the affected area was almost extirpated and was replaced by a genetically distinct lagoon-spawning population from an adjacent brackish lake. However, the pattern of gene flow was stable for populations, with unique admixture proportions in local populations despite the high gene flow (F ST = 0.0184). Our results indicate that Pacific herring in Japan spawn in a range of salinities and exchange genes between local populations regardless of the spawning ecotypes. We hypothesize that the combination of constant gene flow between local populations from straying of spawners and spawning fidelity creates weak but significantly differentiated stable population structure. This process can allow restoration of the genetic characteristics of damaged populations over many generations and can thereby promote the long-term viability of marine fishes that have high gene flow.
Similar content being viewed by others
Introduction
Environmental disturbance is a major driver of population dynamics that has shaped the diversity of many of the world’s ecosystems (Sousa 1984; Turner 2010; Banks et al. 2013). The genetic diversity within species influences biodiversity because of its relationship to individual fitness and viability, population adaptation, and the evolution of new species/subspecies (Sousa 1984; Hughes et al. 2008). Therefore, a natural disturbance that affects genetic diversity can have pervasive ecological and evolutionary consequences (Banks et al. 2013). Disturbances of spatial population structure may also influence the viability of populations. Previous studies have evaluated the genetic impacts of the Chi–Chi Earthquake in Taiwan on an endangered plant species (Hung et al. 2005), floods on an abundant gastropod species (Evanno et al. 2009) and mice (Vignieri 2010), forest fires on a tailed frog (Spear and Storfer 2010) and a bird species (Suárez et al. 2012), volcanic eruptions on a tailed frog (Spear et al. 2012), and hurricanes on a coastal fish species, sailfin molly (Apodaca et al. 2013). All of these studies reported a decrease or increase in genetic diversity after a natural disturbance, but only one study, of the sailfin molly, detected a change in population structure. Evidence of such effects is sparse because the data are difficult to obtain (Apodaca et al. 2013); thus the influence of natural disturbances on the patterns and distribution of genetic diversity is poorly understood (Banks et al. 2013). The Great Tohoku earthquake (magnitude 9.0), which occurred on March 11, 2011 (Sato et al. 2011; Mori et al. 2011; Tsuji et al. 2014), represents an opportunity to evaluate the effect of a rare natural disturbance event on the genetic diversity and population structure of marine organisms.
Pacific herring was a commercially important species for Japan in the 19th and first half of the twentieth centuries (Watanabe et al. 2008). The Pacific herring catch (MAFF 1979) peaked at 787,000 t in 1913, but declined steadily thereafter and was negligible in the late 1950s (Fig. S1). The highest proportion of the harvest was from the Hokkaido-Sakhalin population, which spawned in high-salinity coastal waters (32–34‰) and migrated across a large range. This oceanic wide-migration-type herring lives 13–16 years and has an age at maturity of 4–5 years (Kobayashi 1993). Currently, herring fisheries target several local stocks that spawn in coastal waters and brackish lagoons along the Pacific coast of northern Japan. The catch began to increase in the 2000s and the present level of harvest is ~4000 t in the major fishing areas of Hokkaido, Aomori, Iwate, and Miyagi Prefectures (MAFF 1967–2016) (Fig. S1). These local herring are classified into spawning ecotypes, including an oceanic small-migration type (hereafter, sea-spawning type) and lagoon small-migration type (lagoon-spawning type), both of which have a shorter lifespan (~8 years) and younger age at maturity of 2–3 years than the oceanic wide-migration type (Kobayashi 1993). Pacific herring spawn exclusively in shallow near-shore habitats and females deposit adhesive eggs directly on the bottom or on vegetative substrata (Haegele and Schweigert 1985). The spawning activity is conspicuous: milt turns the water milky-white (Hay et al. 2009), which had not been observed in Japanese coastal waters since the collapse of the so-called “Hokkaido-Sakhalin spring spawning herring” in late 1970s (Fig. S1), but in recent years it has occurred in Ishikari Bay, Hokkaido. Japanese herring spawn only in spring, and the peak spawning period varies between January and May depending on the spawning site. Return to natal spawning areas (natal homing) and return of spawning fish to the same spawning area (spawning fidelity) have been documented in Pacific herring in British Columbia (Hay et al. 2001) and in Japan (Okouchi et al. 2008).
The funnel-shaped bays along the Pacific coast in the Tohoku region, Honshu Island, amplified the tsunami waves of the Great Tohoku earthquake, generating extensive run-up (Seike et al. 2013). Tohoku consists of six prefectures, of which Aomori, Iwate, Miyagi, and Fukushima prefectures border the Pacific coast. The observed tsunami height distribution (Japan Meteorological Agency 2012) indicates that Iwate and Miyagi Prefectures were at the center of the tsunami (Fig. 1a). The maximum run-up height was over 20 m (Tsuji et al. 2014) and was as high as 39.7 m (Mori 2011) in Miyako Bay, Iwate Prefecture. The peak spawning period of the Pacific herring is January to early April in the Tohoku area. Therefore, herring had likely already spawned large quantities of fertilized eggs on seaweed beds or the bottom when the mega-thrust earthquake occurred. It is highly unlikely that the eggs or larvae were able to survive the tsunami. After the earthquake, the catch declined in the major fishing areas of Tohoku, including in Aomori, Iwate, and Miyagi prefectures (Fig. S1). Thus, the reproductive success of the herring populations in damaged areas may have been seriously affected. In contrast, catch volumes increased in Hokkaido after the earthquake.
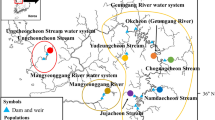
Spatiotemporal population structure of Japanese Pacific herring. a Sampling sites in the major spawning grounds in Hokkaido and Tohoku district with the tsunami height distribution. b STRUCTURE bar plot (K = 7) given the location information of the spawning grounds. c Neighbor-joining tree based on the pairwise F ST values. Numbers after the sample abbreviations indicate sampling year for temporal samples (Table 1). (Color figure online)
We evaluated the effect of the Great Tohoku earthquake on Pacific herring populations using samples collected before and after the earthquake throughout the range of distribution in Japan. We used our historical datasets of microsatellite genotypes of Pacific herring (Clupea pallasii) (Sugaya et al. 2008; Nemoto et al. 2008, and unpublished data) collected in the range of Pacific herring distribution in Japan over a period of 10 years prior to the earthquake, including in the area affected by the tsunami. Additionally, we collected new samples after the earthquake and genotyped them at the same microsatellite loci. We show that the population structure is spatiotemporally stable even after the megathrust earthquake, except in the center of the area affected by the tsunami. Our results demonstrate how a megathrust earthquake influences the patterns and distributions of genetic diversity and population structure of a marine fish. Our results highlight the importance of the stable population structure, which may contribute to the sustainability of widely distributed marine fish populations.
Materials and methods
Sample collection and biological measurements
We used a before–after control-impact (BACI) design (Smith 2002) to isolate the effect of the tsunami. We collected samples of herring (n = 4466 individual fish) during 20 sampling events spread among nine major spawning grounds in Japan during peak spawning periods between 2003 and 2014. Fisheries target mature herring migrating to spawn in bays and lagoons by using gill nets and set nets. Therefore, we could collect samples from the spawning grounds correctly by purchasing herring at the fish markets where the spawning herring landed and/or directly from fishermen. The sampling sites included six locations in Hokkaido, and three locations in Tohoku district (Table 1; Fig. 1a).
Before the Great Tohoku earthquake, we collected samples during 17 sampling events between 2003 and 2008. Samples were collected from landings in Ishikari Bay (IK), Lake Saroma (SR) and Lake Notoro (NT) in 2006, Lake Akkeshi (AK03 and AK06) in 2003 and 2006, Lake Yudo Numa (YD03 and YD07) and Funka Bay (FK) in 2003 on Hokkaido Island, and Lake Obuchi-numa in Aomori Prefecture (OB04, OB05, OB06, and OB07), Miyako Bay in Iwate Prefecture (MY04, MY05, MY07, and MY08) and Matsushima Bay in Miyagi Prefecture in 2005 (MT) in the Tohoku district on Honshu Island. These samples represent the controls as they were collected before the earthquake, including in the affected areas. Additionally, we used samples of recaptured hatchery-reared fish from Lake Akkeshi (AKH) collected in 2003 and Miyako Bay (MYH) in 2005. The otoliths of all hatchery fish were chemically marked before release.
After the earthquake, we collected herring in the spawning season from spawning ground at the center of the area affected by the tsunami, including from Miyako Bay (MY13) in 2013 and Lake Obuchi-numa (OB14) in 2014, and one sample from Lake Akkeshi (AK13). Very few herring spawned in Miyako Bay in 2013, and only 657 fish were caught between late January and early April. Based on otolith aging, these were primarily 2- and 3-year-old fish. We selected all 93 2-year-old individuals (MY13) caught between January 29 and March 7 for genotyping to examine the effect of the earthquake on the 2011 spawning population because these individuals were spawned the year of the earthquake and first spawned at age 2 years in 2013. The muscle tissues of MY13 samples were kindly provided from Miyako Station, Tohoku National Fisheries Research Institute. We also selected 118 2-year-old fish caught in Lake Akkeshi and Akkeshi Bay between April 24 and May 8 (AK13) for genotyping. In Lake Obuchi-numa, only five fishermen catch herring in a very short fishing period of a week-10 days before herring go out from the lake after spawning. We purchased herring caught in Lake Obuchhi-numa during April March 17–18, 2014 (OB14) when they first migrated into the lake not to miss not to miss the chance of sampling. All fish had mature gonads and were 2 or 3 years old; thus, we used all of these fish for genotyping to evaluate the direct effects of the tsunami on the population structure of fish in the spawning grounds in 2013 and the possible remaining effects in 2014. The peak spawning period is late January to early April in Miyako Bay, mid-April to mid-May in Lake Akkeshi and Akkeshi Bay, and late February to mid-March in Lake Obuchi-numa where herring can only migrate into the lake after the adjoining creek thaws and the lake is connected to the sea. Taking onto account the spawning periods and the tsunami height distribution (Fig. 1), we categorized AK13 as a control sample and MY13 and OB14 as impact samples.
For each individual, we measured total length (TL) and/or fork length or body length (BL), body weight (BW), and gonad weight (GW) (n = 4466). The mean body length ± standard deviation (SD) of our samples was 26.5 ± 2.8 cm, and BW was 168.7 ± 62.2 g (Fig. S2). Additionally, we calculated the gonadosomatic index (GSI = GW/BW × 100) and the condition factor (CF = BW/TL3 × 100) for each individual. Almost all fish had mature gonads, but some were already spent (Fig. S2, Table S1). The female ratio was 0.47 ± 0.10. The mean gonad weight was 14.2 ± 7.8 g, and GSI was 14.8 ± 7.8. The mean CF was 0.86 ± 0.11. Ages were determined by examining otoliths (n = 4410), except for the samples collected in Lake Obuchi-numa in 2014 (OB14) for which the age was determined based on comparison of BL with the BL/age distribution relationship of historical samples, which consisted of 2- and 3-year-old fish. The age composition was: 0.3% 1-year-old fish (13 immature fish in YD03 samples), 67.3% 2-year-old fish, 29.8% 3-year-old fish, and 2.6% 4-year-old fish.
Genotyping and quality check
Muscle tissue from each specimen was stored in 99.5% ethanol for DNA extraction. Genomic DNA was extracted following the standard phenol–chloroform procedure and/or use of the QuickGene Mini-80 (Wako Pure Chemical, Osaka, Japan), according to the manufacturer’s instructions. We genotyped all DNA samples (n = 3784) at five microsatellite loci (Cha17, Cha20, Cha63, Cha113, and Cha123) (O’Connell et al. 1998). The annealing temperatures were 57 °C for Cha17, Cha20, and Cha63, and 52 °C for Cha113 and Cha123. For the historic samples, we used observed allele scores (bp) of AK03, AKH, YD03, and FK (first group, n = 1006), which we genotyped using an ABI 3100 Genetic Analyzer (Sugaya et al. 2008). We also used those of IK, SR, NT, AK06, YD07, OB05, OB06, OB07, MY05, MYH, MY07, MY08, and MT from 2006 to 2008 (second group, n = 2477), which we genotyped using an ABI 3130xl (Nemoto et al. 2008). Among these historical genotypes, 54% were unpublished (n = 1331). We genotyped individuals for the after-impact samples from AK13, OB14, and MY13 (n = 301) with the same protocol using an ABI 3130xl. To standardize allele size-shifts observed in the allele size distributions between the new and historic samples, we conducted an inter-laboratory standardization (Ellis et al. 2011). We used the second group as a standard because the sample size (n = 2477) was the largest among groups (65%), which should provide confidence modes in the allele distributions. We added 1.6 and 10.6 to raw allele scores of the first group and the new samples at Cha17, 1.8 and 5.0 at Cha20, 2.8 and 8.0 at Cha63, 2.0 and 7.8 at Cha113, and 4.0 and 2.0 at Cha123. The calibration provided consistent allele score distributions for the three groups at each locus, from which we fixed allele sizes for all individuals. Genotype data quality was evaluated using Microchecker (Van Oosterhout et al. 2004) to detect scoring errors and null alleles. All genotypes used in this study were deposited on the DRYAD (doi:10.5061/dryad.jm60b).
Population genetics analyses
Linkage disequilibrium was tested using GENEPOP 4.2 (Raymond and Rousset 1995) with a 5000 dememorization number, 100 batches, and 5000 iterations per batch. Hardy–Weinberg equilibrium (HWE) was tested using GENEPOP with 10,000 dememorizations, 100 batches, and 10,000 iterations. In these tests, we controlled familywise error rate (FWER) by Bonferroni correction. Allelic richness and heterozygosity were calculated for each locus and population using FSTAT 2.9.3.2 (Goudet 1995) and Arlequin 3.5.1.3 (Excoffier et al. 2005), respectively. Samples were rarefied to the smallest sample size to determine allelic richness (n = 34). The exact test for differences in allele frequencies was performed using the Markov chain procedure (5,000 dememorization number, 100 batches, and 5,000 iterations), as implemented in GENEPOP.
We estimated the maximum likelihood global F ST over all populations using EBFST1.2 (http://www.g.kaiyodai.ac.jp/cmls1/Conservation) (Kitada et al. 2007), from which we calculated the rate of gene flow for the Wright island model as: \(\theta =1/{{\bar{F}}_{\text{ST}}}-1\) (Wright 1951), where \({{\bar{F}}_{\text{ST}}}\) is the simple mean F ST over all loci and θ is the scale parameter of a Dirichlet distribution. For diploid populations, \(\theta =4{{N}_{e}}m\). Here, \({{N}_{e}}\) is the effective population size and m is the migration rate in each generation. The composite parameter \({{N}_{e}}m\) indicates the number of individuals replaced by migrants per population per generation. Therefore, θ represents the rate of gene flow. We tested the null hypothesis H0: homogeneity of \({{F}_{\text{ST}}}\) among loci against the alternative hypothesis H1: heterogeneity of \({{F}_{\text{ST}}}\) among the loci (Kitada et al. 2007). In this test, we excluded hatchery fish populations (AKH and MYH) to avoid the possible effect of artificial selection.
We ran STRUCTURE 2.3.4 (Pritchard et al. 2000) with the admixture linkage disequilibrium model (Falush et al. 2003) using a burn-in of 100,000 iterations followed by 500,000 Monte Carlo–Monte Carlo repetitions for the number of putative original populations K = 1–10. We then ran STRUCTURE using the LOCPRIOR option, which considers geographical information (Hubisz et al. 2009). As natal homing and spawning fidelity are known in herring, and all samples were collected from the spawning populations, the nine sampling localities would provide meaningful information on population differentiation. Therefore, we assigned the integers 1–9 to the nine sampling locations. We estimated F ST between pairs of populations using EBFST to obtain precise F ST estimates for high gene flow species such as marine fish. We drew a neighbor-joining unrooted phylogenetic tree (Saitou and Nei 1987) using FigTree (http://tree.bio.ed.ac.uk/software/figtree/) based on the pairwise F ST values.
We estimated the effective population size (\({{N}_{e}}\)) based on the temporal change in allele frequencies (Nei and Tajima 1981; Pollak 1983; Waples 1989) for Lake Akkeshi, Lake Obuchi-numa, and Miyako Bay using NeEstimator (Do et al. 2014). The multilocus F k (Pollak 1983) was used for the F-statistic. If \({{N}_{e}}\) changes with time, the estimate is the harmonic mean of the effective population sizes in the individual generations (Nei and Tajima 1981; Pollak 1983). We calculated the mean age at spawning from individual ages determined by otolith examination of all samples, excluding the AK13, OB14, and MY13 after-impact samples because we selected 2-year-old fish for AK13 and MY13, and 2–3-year-old age fish for AK13. The 13 1-year-old immature fish in the YD03 sample were also excluded. The mean age at spawning was 2.35 ± 0.53 (SD) (n = 4410, otolith examined 2–4-year-old fish), which was used as the generation time (t). The generation length (GL) between samples was calculated (GL = years between samples/t). Generation time (t) is the crucial parameter in estimating GL. In our case, t = 2.35, whereas t = 5.25 for Atlantic herring (Larsson et al. 2010) that have much greater longevity (~22 years) and older ages at maturity (3–9 years) (Beverton et al. 2004) than the Japanese local herring (~8 and 2 years) (Kobayashi 1993). Our data show that the age at maturity of the Japanese local herring was 2 years (Table S1), and no fish older than 4 years were collected over the last decade. Our GL values based on the mean age at spawning should not have serious bias.
To evaluate the effect of the Great Tohoku earthquake on the herring population in the center of the tsunami, we estimated the mixing proportions of local populations in Miyako Bay and in Lake Obuchi-numa before and after the earthquake using ONCOR (Kalinowski et al. 2007), which was used in a study of Atlantic herring (Bekkevold et al. 2011). We assumed nine baseline populations in the major spawning grounds as shown in the “Results”: IK, SR, NT, AK, YD, FK, OB, MY, and MT. For the pre- and post-tsunami samples from MY and OB we used all samples available from previous years, to determine which populations were contributing to the spawning aggregations. The 95% confidence intervals (CI) for the mixing proportion estimates were calculated with 1000 bootstraps in ONCOR. We then estimated the change in the commercial catch composition of the original MY and OB populations before and after the earthquake. We multiplied the commercial catch of 1.495 t for 2008 (0.215 t for 2013) in Miyako Bay by the estimated mixing proportions for the MY08, and MY13 samples. We used commercial catch values of 0.850 t for 2007 and 2.175 t for 2014 to estimate the catch compositions in Lake Obuchi-numa in these years. Using this information, we calculated the catch by weight for individuals coming from the original baseline populations in Miyako Bay and in Lake Obuchi-numa before and after the earthquake. The calculated catches estimate the changes in the number of individuals come from the baseline populations, which can be used to infer rates of gene flow between local populations.
Results
We obtained the genotypes of 3,784 fish collected during 20 sampling events spread among nine major spawning grounds in Japan during peak spawning periods between 2003 and 2014 (Table 1). A total of 157 alleles were found at the five microsatellite loci, and no evidence was detected for scoring errors caused by stuttering, large allele dropout, or null alleles. All pairs of loci were in linkage equilibrium in all samples, except for some pairs of loci after Bonferroni correction (Table S2).
The global F ST values for the overall population at each locus were 0.0171 ± 0.0014 (Cha 17), 0.0168 ± 0.0019 (Cha 20), 0.0152 ± 0.0016 (Cha 63), 0.0229 ± 0.0024 (Cha 113), and 0.0198 ± 0.0015 (Cha 123), respectively. The Cha 113 locus had a higher value than those of other loci, and the homogeneity of F ST was rejected (\(\lambda =9.4982,\) P = 0.0498). However, when we excluded the after-impact sample collected from Miyako Bay (MY13), F ST homogeneity was supported (\(\lambda =8.0811\), P = 0.0887).
A cluster analyses suggested that the most likely number of putative original populations was K = 7 with location information of the spawning grounds, but K = 6 without it (Table S3, Fig. 1b, S3). The bar plots for both models revealed a similar pattern among the major spawning grounds, with unique admixture proportions of the original populations. All local populations were in HWE (Fig. 2, Table S4). As discussed in Discussion, K = 7 was supported by earlier studies. The F ST values between pairs of populations were 0.0120 ± 0.0052 (SD) (Table 2), and the neighbor-joining unrooted trees revealed three large clusters representing Hokkaido, Lake Obuchi-numa (OB), and Miyako Bay (MY)/Matsushima Bay (MT) (Fig. 1c). The Hokkaido cluster consisted of six HWE populations, Ishikari Bay (IK), Lake Saroma (SR), Lake Notoro (NT), Lake Akkeshi (AK), Lake Yudo-numa (YD), and Funka Bay (FK). YD and FK looked very distinct from the other populations. The clockwise geographic structure beginning with Ishikari Bay was connected first to the Lake Obuchi-numa cluster, and then to those of Miyako Bay and Matsushima Bay. The population structure and admixture patterns of local populations were stable in Hokkaido and Lake Obuchi-numa, even after the tsunami (Fig. 1). However, MY13, collected from the area at the center of the tsunami, had a different admixture proportion from those of the before-impact samples in Miyako Bay, though it was similar to those from Lake Obuchi-numa (Fig. 1b, S3), and was included in the Lake Obuchi-numa cluster (Fig. 1c).
The allele frequencies differed significantly between populations, except for comparisons between before-impact MY and MT, and NR and SR samples, and within the before-impact samples from Lake Obuchi-numa, Miyako Bay, and Lake Akkeshi (Table 2). The allele frequencies of the after-impact AK13 sample did not differ from the before-impact AK06 sample. In contrast, allele frequencies from MY13 and OB14 clearly differed from their respective before-impact samples (Table 2, Fig. S4). Interestingly, the MY13 allele frequencies were not different from those of the OB05, OB06, and OB07 before-impact samples, but differed slightly from those of OB14, although the distributions of allele frequencies were similar. The allele frequency distribution was consistent with the population structure (Fig. 1b, c).
Genetic diversity was consistently high in fish from Hokkaido but low in those from Tohoku, as shown by the allelic richness values, which were 16.58 ± 4.64 and 11.67 ± 2.61 (Table 3). Allelic richness and observed heterozygosity were not different in Lake Akkeshi and in Lake Obuchi-numa between samples collected before and after the earthquake. Unexpectedly, heterozygosity increased in Miyako Bay after the earthquake and allelic richness tended to increase but was not significant. The \({{N}_{e}}\) estimates for local populations were generally small, ranging from 37 [26, 51] to 746 [341, 4,450] (Table 4). The \({{N}_{e}}\) increased in Lake Akkeshi after the earthquake, from 124 [87, 178] to 746 [341, 4450]. Conversely, there was a substantial decrease in \({{N}_{e}}\) in Miyako Bay from 261 [149, 504] to 37 [26, 51] (14%) but an increase in \({{N}_{e}}\) in Lake Obuchi-numa from 114 [60, 254] to 183 [97, 405].
In Miyako Bay, the mixing proportion estimate of the original Miyako Bay population (sea-spawning type) decreased substantially from 79.0% (2008) to 4.4% after the earthquake (2013). Similarly, the estimate for the Matsushima Bay population (sea-spawning type) also decreased from 13.7 to 2.4%. In contrast, the estimate from Lake Obuchi-numa (lagoon-spawning type) increased significantly from 6.9 to 78.5% (Fig. 3a Left, Table 5). We observed low frequency, but consistent migration from Ishikari Bay and from Lake Akkeshi (10.4%, 456 km linear distance) after the earthquake. Commercial catch decreased from 1.495 t (2008) to 0.215 t (2013) in Miyako Bay. The estimated catch data suggests that the original Miyako Bay population (1.180 t in 2008) had a very low contribution to catch in 2013 (0.009 t) (Fig. 3a, Middle, Table S5). A similar pattern was observed for the original Matsushima Bay population, which decreased from 0.205 t to 0.005 t. Conversely, the catch of individuals from the original Lake Obuchi-numa population (160 km) was relatively constant, but increased from 0.103 t to 0.169 t.
The composition of local populations in a Miyako Bay and b Lake Obuchi-numa before and after the Great Tohoku earthquake. Mixing proportions of the local populations (left), and catch compositions based on the mixing proportion estimates and catch statistics (middle). The bars represent 95% confidence intervals. Geographical features (right). Yellow and blue lines in the maps represent major roads and rivers, respectively. (Color figure online)
The catch proportion of the local populations were stable in Lake Obuchi-numa even after the earthquake, with 84–90% being the original Obuchi-numa population with small but consistent immigration from Miyako Bay (1–3%) and Lake Akkeshi (7–11%) (Fig. 3b Left, Table 6). The commercial catch doubled from 0.850 t (2007) to 2.175 t (2014) in Lake Obuchi-numa, resulting in a 2.4 fold increase in the catch of individuals from the original Lake Obuchi-numa population from 0.763 t in 2007 to 1.827 t in 2014 (Fig. 3b, Middle, Table S5). However, immigration from the original Lake Obuchi-numa population into Miyako Bay was relatively stable at ~9–14% before (0.135 = 0.103 t/0.763 t) and after (0.093 = 0.169 t/1.827 t) the earthquake.
Discussion
We found that the Japanese herring population consisted of three genetically distinct large clusters: Hokkaido, Lake Obuchi-numa, and Miyako Bay/Matsushima Bay. The pattern of gene flow between local populations was temporarily stable even after the earthquake and exhibited a unique admixture proportion. Our analyses also revealed that the sea-spawning population in Miyako Bay, the area at the center of the tsunami, was almost extirpated and was replaced by a genetically distinct lagoon-spawning herring population in the adjacent brackish Lake Obuchi-numa.
Estimated number of putative original populations with spawning location information (K = 7) was supported by earlier studies using allozymes. Kobayashi (1993) reported two wide-migration types of herring in the coasts of Hokkaido; Hokkaido-Sakhalin (wide-migration-sea-spawning type), and DeKastri in Sakhalin (wide-migration-lagoon-spawning type) populations. Our results suggest that these two Sakhalin populations admixed in Hokkaido (IK, SR, NT, AK, and YD) as shown in red and blue colors (Fig. 1b). The distinct admixture patterns in YD (as shown in orange) and FK (light blue) suggest two putative original populations in Eastern and Western Pacific coast of Hokkaido. YD was isolated AK and populations in the adjacent Nemuro Straight (Hotta et al. 1999). In Honshu Island, the admixture proportions in OB, MY and MT imply three putative original populations of Aomori (yellow), Iwate-Miyagi (green), and the southern Tohoku population (pink), which was well supported by Kijima et al. (1992), who found that samples collected from MY and three locations in Miyagi prefectures included in a cluster, and isolated from Lake Hinuma population (Ibaraki prefecture). This small population was the southern limit of this species with the catch ranged 10–72 t in 1960s, but no catch has been reported since 1999 and might be extinct (MAFF 1967–2016). There might be small spawning in coastal areas between MY and OB, but major spawning grounds have not been observed. Therefore, we did not consider unsampled populations in Honshu. Thus, we concluded that the seven putative original populations were admixed in the spawning grounds, and create Japanese local populations. The low level of genetic diversity of the Tohoku populations should cause three large clusters (Hokkaido, OB, and MY/MT), which was consistent with our previous results (Sugaya et al. 2008; Nemoto et al. 2008).
The F ST value in the Japanese herring populations (F ST = 0.0184) was one order higher than that of other Pacific and Atlantic herring populations as shown below (F ST = 0.002–0.008). As megathrust earthquakes occur at intervals of 500–800 years in northeastern Japan (Sawai et al. 2012), Japanese herring in the Tohoku Pacific coast have likely experienced repeated disturbances by tsunamis. We speculate that the higher level of genetic differentiation of the Tohoku populations and their lower genetic diversity (Table 3) may be a result of recurring megathrust earthquakes. Japanese herring are at the southern periphery of the range of this species, and our effective population size estimates (\({{N}_{e}}\)) were one order of magnitude or greater below estimates for the major populations of Atlantic herring (Larsson et al. 2010). The causal mechanisms for population structuring are migration and genetic drift, and the magnitude of differentiation depends on the number of migrants (\({{N}_{e}}m\)) (Hauser and Calvalho 2008; Waples and Gaggiotti 2006). A smaller \({{N}_{e}}\) is subject to larger genetic drift and lower numbers of migrants, resulting in larger F ST values. Bias in the \({{N}_{e}}\) estimate for species with overlapping generations can be substantial, but in many cases bias largely disappears if samples are taken 5–10 generations apart (GL = 5–10) (Waples and Yokota 2007). In our analysis, GL = 1.28–4.26. Based on Fig. 4 of Waples and Yokota, the bias was negative (~12.5–20%) for a one to four generation interval when 100 barnacles were randomly collected at each sampling event, conditions that match those in our study. We may have underestimated \({{N}_{e}}\) values by, at most, ~20%; however, this margin would not be enough to change the overall conclusions of the study.
Our results suggest that the pattern of gene flow was stable in Japanese herring populations even after the megathrust earthquake. Such stable but high gene flow population structures were found in Atlantic herring in Swedish waters (F ST = 0.002–0.003, Larsson et al. 2010) and in Pacific herring in British Columbia and adjacent regions (F ST = 0.003, Beacham et al. 2008). Slightly larger genetic differentiation was found in North Sea-Baltic Sea Atlantic herring (F ST = 0.008, Bekkevold et al. 2005, F ST = 0.0073; Jørgensen et al. 2005). Temporally stable and significantly differentiated structures with high gene flow were also found in major Atlantic cod (Gadus morhua) populations (F ST = 0.0012–0.0400, Poulsen et al. 2006) even over small geographic scales (F ST = 0.0023, Knutsen et al. 2003) as reviewed by Hauser and Calvalho (2008). Chemical marking of otoliths previously revealed that 53–70% of herring that migrate to Miyako Bay for spawning were hatchery-reared progeny of wild herring that migrated to Miyako Bay and were released at a TL of 5 cm in Miyako Bay (Okouchi et al. 2008). Additionally, our analysis of otolith marks revealed that the hatchery fish samples (AKH and MYH) were released and recaptured as mature fish (aged 2–4 years) in Lake Akkeshi and Miyako Bay. Previous tagging experiments using external tags also found that wild herring that spawned in Miyako Bay in February migrated to Funka Bay (linear distance: 352 km) in June–September for feeding and returned to Miyako Bay in the next spawning season; though the same experiments found that some fish strayed into other spawning grounds in Iwate and Miyagi Prefectures (Okouchi et al. 2008). Together, these observations suggest a high degree of natal homing and spawning fidelity, but not necessarily to natal spawning areas (Beacham et al. 2008), and some fish do stray to other spawning grounds. Evidence for spawning fidelity in marine fish has been reported in Atlantic cod (Green and Wroblewski 2000; Wright et al. 2006; Skjæraasen et al. 2011) even for long distance (Bonanomi et al. 2016), North Sea plaice (Pleuronectes platessa) (Hunter et al. 2003), and bluefin tuna (Thunnus thynnus thynnus) in the western Atlantic (Nemerson et al. 2000). All of our samples were collected in the spawning grounds and almost all fish were mature, suggesting that the stable pattern of gene flow was the result of straying of spawning fish. Levels of natal homing, straying, and spawning fidelity should define the migration rate, which can vary over generations, but will converge to the population mean when the number of generations approaches infinity by the law of large numbers, providing a constant gene flow. This may be a mechanism for temporally stable structure in marine fishes with high gene flow.
Differences in the effect of the tsunami on herring at different sites may be a function of the geography of the spawning grounds. Miyako Bay (24 km2, maximum depth, 60 m) is a funnel-shaped, semi-open bay with a 4-km-wide bay mouth (Fig. 3), which likely amplified the height of the tsunami. In contrast, Lake Obuchi-numa is a closed lagoon (3.7 km2, maximum depth, 4.5 m) connected to the sea by a 20-m-wide and 1.5-km-long creek. The water level increased ~ 1 m in Lake Obuchi-numa during the tsunami but there was no substantial damage to the fishery (Rokkasho-mura Fisheries Cooperative, Pers. Comm.). Therefore, even if herring were spawning on March 11 or had spawned eggs in Lake Obuchi-numa, they were probably protected from a damaging tsunami by the geography of the lake. Thus, these herring may have acted as a source for rebuilding the Miyako Bay herring population. In contrast, Lake Akkeshi is a lagoon (32 km2, maximum depth: 11 m) that is directly connected to the Akkeshi Bay through a 500-m-wide lake mouth. The maximum tsunami height was 3.5 m in the bay, which was sufficient to destroy manila clam (Venerupis philippinarum) and Japanese oyster (Crassostrea gigas) aquaculture facilities in the lake. The size of the tsunami was sufficient to affect survival of herring eggs; however, it occurred before the peak spawning period in Lake Akkeshi (mid-April to mid-May) and is therefore unlikely to have had a large effect on the reproductive success of the Akkeshi herring. Indeed, the catch of herring in the spawning season increased from 27 t in 2010 to 116 t in 2011 in Lake Akkeshi, and reached a post-1970 maximum of 1,240 t in 2016 (Fig. 4). Consistent with this, our \({{N}_{e}}\)estimate (746) increased six-fold over this period. The rapid increase in harvest volume suggests that the tsunami may have had a positive effect on the spawning ground habitat, though it is unclear why the effective population size increased. In contrast, harvest decreased by ~50% (from 2.9 t in 2010 to 1.5 t in 2012) in Lake Obuchi-numa, but rebounded (2.6 t) in 2015 and remained high (2.3 t) in 2016 (Fig. 4). The catch in Miyako Bay increased to 0.40 t in 2014 but then decreased to 0.12 t in 2015. Fishing operations resumed 1 month after the earthquake in Miyako Bay, and the number of small set nets targeting several fish species, including herring, was similar to pre-earthquake levels by 2015. Thus, we speculate that this substantial fishing pressure contributed to the decline in the recovering population after 2014. However, catch rebounded to 0.47 t in 2016 (Fig. 4), suggesting the tsunami had a positive effect on spawning ground habitat.
The admixture proportion in Miyako Bay (sea-spawning type) suggests there was constant migration of spawners from Lake Obuchi-numa (lagoon-spawning type) before the earthquake (Fig. 1b). We genotyped MY13 in 2013, and OB14 in 2014. Therefore, there was no chance for substitution or contamination of these samples. Our mixed catch analysis revealed that stable gene flow (~9–14%) from Lake Obuchi-numa into the decimated Miyako Bay population resulted in the substantially altered admixture proportion in MY13. The sample we used for the calculation of mixed catches were collected in several landing days during the spawning season in MY; MY08 (Feb. 22–Mar. 21), and MY13 (Jan. 29–Apr. 2) (Table S1). The spawning period is very short (a week-10 days) in Obuchi-numa, and we bought all fish caught by a fisherman in a short period; OB07 (Feb. 19, n = 98), and OB14 (Mar. 17–18, n = 112). However, HWE was hold in all populations. Therefore, we could assume that our samples represented genotypes of the populations, and be reasonable to use for the mixed catch analysis.
The \({{N}_{e}}\) estimate in Miyako Bay decreased substantially after the earthquake. This should be caused by altered allele frequencies due to population decimation and the constant gene flow from Lake Obuchi-numa. Therefore, the estimate suggested a decrease in the effective population size in Miyako Bay. Our \({{N}_{e}}\) estimate in Lake Obuchi-numa was 158 ± 38 (SD), of which 9–14% of gene flow accounts for 14–22 individuals per generation, which is consistent with our \({{N}_{e}}m\) estimate (~14 = 54.5/4). The Miyako Bay and Matsushima Bay populations remained, and the gene flow from Hokkaido was low but consistent even after the earthquake (Fig. 3a). Therefore, a full recovery of the admixture proportion may be expected in Miyako Bay; however, it may take many generations. Conversely, the temporarily stable admixture proportion (Fig. 1b) and the stable pattern of mixing proportion (Fig. 3b) in Lake Obuchi-numa indicates there was constant gene flow from Miyako Bay, even after the earthquake. Given that the earthquake decimated the Miyako Bay population, this gene flow may be a result of the spawning fidelity of fish straying from Miyako Bay before the earthquake (previous generations). Our results suggest that this constant gene flow can restore the genetic characteristics of damaged populations over many generations. The time until recovery from the most recent disturbance (2011) will depend on the rate of gene flow, with higher gene flow resulting in a shorter time for recovery. The stable spatial structure of populations and the stable pattern of gene flow may represent a mechanism for the long-term viability of fish populations by allowing marine fishes with high gene flow to exhibit plasticity in their response to environmental disturbances.
Salinity and sea temperature during the spawning season were 9–11‰ and 6 °C in Lake Obuchi-numa, whereas they were 30.8‰ and 8.1 °C in Miyako Bay. Sea-spawning-type herring spawn in Ishikari Bay (33‰, 6–7 °C) (Kobayashi 1993). In contrast, lagoon-spawning-type herring spawn simultaneously in Akkeshi Bay and Lake Akkeshi. Salinity is generally low in Lake Akkeshi during the peak spawning season in April (15–22‰, 8–12 °C) because of sea thawing, but high (30–32‰) outside the lake (Kakuda 1997). Similarly, Lake Notoro (16–27‰, 5–18 °C) and Lake Saroma (19–22‰, 9–10 °C) (Kobayashi 1993) have low salinities in the spawning season, but high salinities at other times because of their direct connection to the sea. In contrast, the salinity is very low (~4‰) in Lake Yudo-numa, which is usually closed to the sea because of a 4-km sand bar, but does connect occasionally during sea thaws or heavy rains. Pacific herring have a lower level salinity tolerance (0–6‰) during early development (McMynn and Hoar 1953). Lowered salinity caused by sea thaws may be a driver of spawning behavior of Pacific herring. Salinity ranges for the lagoon-spawning (9–27‰) and sea-spawning (31–33‰) herring in Japan are consistent with estimates for Pacific herring (8–28‰) in North America (Alderdice and Velsen 1971). Atlantic herring spawn in a wider range of salinity, including in the Baltic Sea (3.5–9.7‰), the Baltic–North Sea transition area (16.0–31.2‰), and the North Sea (34.0–35.2‰) (Bekkevold et al. 2005; Gaggiotti et al. 2009; Limborg et al. 2012). The Northeast Atlantic herring form three, large, divergent genetic clusters, which have been detected even when using the putatively neutral 265 SNP markers (Limborg et al. 2012). Our \({{F}_{\text{ST}}}\) homogeneity test suggests that altered allele frequencies in MY13 inflated heterogeneity of the F ST, and indicates that the microsatellite loci used in this study may be neutral but not involve locally adaptive genes. Our results show that Pacific herring in Japan spawn in a range of salinities and exchange genes between local populations, regardless of whether they are lagoon-spawning or sea-spawning.
References
Alderdice DF, Velsen FP (1971) Some effects of salinity and temperature on early development of Pacific herring (Clupea pallasi). J Fish Board Can 28:1545–1562
Apodaca JJ, Trexler JC, Jue NK, Schrader M, Travis J (2013) Large-scale natural disturbance alters genetic population structure of the Sailfin Molly, Poecilia latipinna. Am Natl 181:254–263
Banks SC, Dujardin M, McBurney L, Blair D, Barker M, Lindenmayer DB (2013) How does ecological disturbance influence genetic diversity? Trends Ecol Evol 28:670–679
Beacham TD, Schweigert JF, MacConnachie C, Le KD, Flostrand L (2008) Use of microsatellites to determine population structure and migration of Pacific herring in British Columbia and adjacent regions. Trans Am Fish Soc 137:1795–1811
Bekkevold D, Andre C, Dahlgren TG, Clausen LAW, Torstensen E, Mosegaard H, Carvalho GR, Christensen TB, Norlinder E, Ruzzante DE (2005) Environmental correlates of population differentiation in Atlantic herring. Evolution Int J org Evolution 59:2656–2668
Bekkevold D, Clausen LA, Mariani S, André C, Hatfield EM, Torstensen E, Ryman N, Carvalho GR, Ruzzante DE (2011) Genetic mixed-stock analysis of Atlantic herring populations in a mixed feeding area. Mar Ecol Prog Ser 442:187–199
Beverton, RJH, Hylen A, Østvedt OJ, Alvsvaag J, Iles TC (2004) Growth, maturation, and longevity of maturation cohorts of Norwegian spring-spawning herring. ICES J Mar Sci 61:165–175
Bonanomi S, Overgaard Therkildsen N, Retzel A, Berg Hedeholm R, Wæver Pedersen M, Meldrup D et al. (2016) Historical DNA documents long distance natal homing in marine fish. Mol Ecol 25:2727–2734
Do C, Waples RS, Peel D, Macbeth GM, Tillett BJ, Ovenden JR (2014) NeEstimator V2: re-implementation of software for the estimation of contemporary effective effective population size (Ne) from genetic data. Mol Ecol Resour 14:209–214
Ellis JS, Gilbey J, Armstrong A, Balstad T, Cauwelier E, Cherbonnel C et al (2011) Microsatellite standardization and evaluation of genotyping error in a large multi-partner research programme for conservation of Atlantic salmon (Salmo salar L.). Genetica 139(3):353–367
Evanno G, Castella E, Antoine C, Paillat G, Goudet J (2009) Parallel changes in genetic diversity and species diversity following a natural disturbance. Mol Ecol 18:1137–1144
Excoffier L, Laval G, Schneider S (2005) Arlequin ver. 3.0: an integrated software package for population genetics data analysis. Evol Bioinf Online 1:47–50
Falush D, Stephens M, Pritchard JK (2003) Inference of population structure using multilocus genotype data: linked loci and correlated allele frequencies. Genetics 164:1567–1587
Gaggiotti OE, Bekkevold D, Jørgensen HBH, Foll M, Carvalho GR, Andre C, Ruzzante DE (2009) Disentangling the effects of evolutionary, demographic, and environmental factors influencing genetic structure of natural populations: Atlantic herring as a case study. Evolution Int J org Evolution 63:2939–2951
Goudet J (1995) Fstat version 1.2: a computer program to calculate F-statistics. J Hered 86:485–486
Green JM, Wroblewski JS (2000) Movement patterns of Atlantic cod in Gilbert Bay, Labrador: evidence for bay residency and spawning site fidelity. J Mar Biol Assoc UK 80:1077–1085
Haegele CW, Schweigert JF (1985) Distribution and characteristics of herring spawning grounds and description of spawning behaviour. Can J Fish Aquat Sci 42:39–55
Hauser L, Carvalho GR (2008) Paradigm shifts in marine fisheries genetics: ugly hypotheses slain by beautiful facts. Fish Fish 9:333–362
Hay DE, McCarter PB, Daniel KS (2001) Tagging of Pacific herring Clupea pallasi from 1936 to 1992: a review with comments on homing, geographic fidelity, and straying. Can J Fish Aquat Sci 58:1356–1370
Hay DE, McCarter PB, Daniel KS, Schweigert JF (2009) Spatial diversity of Pacific herring (Clupea pallasi) spawning areas. ICES J Mar Sci 66:1662–1666
Hotta T, Matsuishi T, Sakano H, Kanno Y (1999) Population structure of Pacific herring Clupea pallasii in the eastern Hokkaido waters. Nippon Suisan Gakkaishi 65:655–660 (in Japanese with English abstract)
Hubisz MJ, Falush D, Stephens M, Pritchard JK (2009) Inferring weak population structure with the assistance of sample group information. Mol Ecol Resour 9: 1322–1332
Hughes AR, Inouye BD, Johnson MT, Underwood N, Vellend M (2008) Ecological consequences of genetic diversity. Ecol Lett 11:609–623
Hung KH, Hsu TW, Schaal BA, Chiang TY (2005) Loss of genetic diversity and erroneous phylogeographical inferences in Lithocarpus konishii (Fagaceae) of Taiwan caused by the Chi-Chi earthquake: implications for conservation. Ann Missouri Bot Gard 92:52–65
Hunter E, Metcalfe JD, Reynolds JD (2003) Migration route and spawning area fidelity by North Sea plaice. Proc Royal Soc Lond B 270:2097–2103
Japan Meteorological Agency (2012) Report on the 2011 off the Pacific coast of Tohoku Earthquake. Tech Rep Jap Meteorol Agcy 133:94–95 (in Japanese with English abstract)
Jørgensen HB, Hansen MM, Bekkevold D, Ruzzante DE, Loeschcke V (2005) Marine landscapes and population genetic structure of herring (Clupea harengus L.) in the Baltic Sea. Mol Ecol 14:3219–3234
Kakuda T. (1997) Water quality in Lake Akkeshi. Kushiro Fish Exp Stn News 76:11–26 (in Japanese)
Kalinowski ST, Manlove KR, Taper ML (2007) ONCOR: Software for Genetic Stock Identification. http://www.montana.edu/kalinowski/Software/ONCOR.htm
Kijima A, Nakajima M, Fujio Y (1992) Genetic differentiation among localities in the natural Pacific herring around Japan and genetic characterization of the artificial seeds compared with the natural populations. Tohoku J Agricult Res 42:83–93
Kitada S, Kitakado T, Kishino H (2007) Empirical Bayes inference of F ST and its distribution in the genome. Genetics 177:861–873
Knutsen H, Jorde PE, André C, Stenseth NCHR (2003) Fine-scaled geographical population structuring in a highly mobile marine species: the Atlantic cod. Mol Ecol 12: 385–394
Kobayashi T. (1993) Biochemical analyses of genetic variability and divergence of populations in Pacific herring. Bull Natl Res Inst Far Seas Fish 30:1–77 (in Japanese)
Larsson LC, Laikre L, André C, Dahlgren TG, Ryman N (2010) Temporally stable genetic structure of heavily exploited Atlantic herring (Clupea harengus) in Swedish waters. Heredity 104:40–51
Limborg MT, Helyar SJ, de Bruyn M, Taylor MI, Nielsen EE, Ogden R et al (2012) Environmental selection on transcriptome-derived SNPs in a high gene flow marine fish, the Atlantic herring (Clupea harengus). Mol Ecol 21:3686–3703
McMynn RG, Hoar WS (1953) Effects of salinity on the development of the Pacific herring. Can J Zool 31:417–432
Ministry of Agriculture, Forestry and Fisheries (MAFF) (1967–2016) Annual statistics of fisheries and aquaculture production in 1965–2014. association of agriculture and forestry statistics, Tokyo (in Japanese)
Ministry of Agriculture, Forestry and Fisheries (MAFF) (1979) Long-term statistics of fisheries production. Association of agriculture and forestry statistics, Tokyo (in Japanese)
Mori N, Takahashi T, Yasuda T, Yanagisawa H (2011) Survey of 2011 Tohoku earthquake tsunami inundation and run-up. Geophysic Res Lett 38 (7)
Nei M, Tajima F (1981) Genetic drift and estimation of effective population size. Genetics 98:625–640
Nemerson D, Berkeley S, Safina C (2000) Spawning site fidelity in Atlantic bluefin tuna, Thunnus thynnus: the use of size-frequency analysis to test for the presence of migrant east Atlantic bluefin tuna on Gulf of Mexico spawning grounds. Fish Bull 98:118–126
Nemoto Y, Sugaya T, Okouchi H, Kitakado H, Hamasaki K, Kitada S (2008) Population structure of Pacific herring Clupea pallasii in the coastal area of Japan, inferred from microsatellite DNA variation. Fish Genet Breed Sci 37:51–59 (in Japanese with English Abstract)
O’Connell M, Dillon MC, Wright JM (1998) Development of primers for polymorphic microsatellite loci in the Pacific herring (Clupea harengus pallasi). Mol Ecol 7:358–360
Okouchi H, Yamane S, Aritaki M (2008) Migration ecology of herring Clupea pallasii after spawning in Miyako Bay and homing in the following year. Nippon Suisan Gakkaishi 74:389–394 (in Japanese with English abstract)
Pollak E (1983) A new method for estimating the effective population size from allele frequency changes. Genetics 104(3):531–548
Poulsen N, Nielsen EE, Schierup MH, Loeschcke V, Grønkjær P. (2006) Long-term stability and effective population size in North Sea and Baltic Sea cod (Gadus morhua). Mol Ecol 15:321–331
Pritchard JK, Stephens M, Donnelly P (2000) Inference of population structure using multilocus genotype data. Genetics 155:945–959
Raymond M, Rousset F (1995) GENEPOP (version 1.2): population genetics software for exact tests and ecumenicism. J Hered 86:248–249
Saitou N, Nei M (1987) The neighbor-joining method: a new method for reconstructing phylogenetic trees. Mol Biol Evol 4:406–425
Sato M, Ishikawa T, Ujihara N, Yoshida S, Fujita M, Mochizuki M, Asada A (2011) Displacement above the hypocenter of the 2011 Tohoku-Oki earthquake. Science 332:1395
Sawai Y, Namegaya Y, Okamura Y, Satake K, Shishikura M (2012) Challenges of anticipating the 2011 Tohoku earthquake and tsunami using coastal geology. Geophysic Res Lett 39:L21309. doi:10.1029/2012GL053692
Seike K, Shirai K, Kogure Y (2013) Disturbance of shallow marine soft-bottom environments and megabenthos assemblages by a huge tsunami induced by the 2011 M9.0 Tohoku-Oki Earthquake. PLOS ONE 8(6):e65417
Skjæraasen JE, Meager JJ, Karlsen Ø, Hutchings JA, Fernö A (2011) Extreme spawning-site fidelity in Atlantic cod. ICES J Mar Sci 68:1472–1477
Smith EP (2002) BACI design. In: Abdel H, El-Shaarawi, Piegorsch WW (eds) Encyclopedia of environmetrics, Wiley, New Yolk, pp 141–148
Sousa WP (1984) The role of disturbance in natural communities. Ann Rev Ecol Syst 15:353–391
Spear SF, Storfer A (2010) Anthropogenic and natural disturbance lead to differing patterns of gene flow in the Rocky Mountain tailed frog, Ascaphus montanus. Biol Cons 143:778–786
Spear SF, Crisafulli CM, Storfer A (2012) Genetic structure among coastal tailed frog populations at Mount St. Helens is moderated by post-disturbance management. Ecol Appl 22:856–869
Suárez NM, Betancor E, Fregel R, Rodríguez F, Pestano J (2012) Genetic signature of a severe forest fire on the endangered Gran Canaria blue chaffinch (Fringilla teydea polatzeki). Cons Genet 13:499–507
Sugaya T, Sato M, Yokoyama E, Nemoto Y, Fujita T, Okouchi H, Hamasaki K, Kitada S (2008) Population genetic structure and variability of Pacific herring Clupea pallasii in the stocking area along the Pacific coast of northern Japan. Fish Sci 74:579–588
Tsuji Y, Satake K, Ishibe T, Harada T, Nishiyama A, Kusumoto S (2014) Tsunami heights along the pacific coast of northern Honshu recorded from the 2011 Tohoku and previous great earthquakes. Pure Appl Geophys 171:3183–3215
Turner MG (2010) Disturbance and landscape dynamics in a changing world. Ecology 91:2833–2849
Van Oosterhout C, Hutchinson W, Wills DP, Shipley P (2004) Microchecker: software for identifying and correcting genotyping errors in microsatellite data. Mol Ecol Notes 4:535–538
Vignieri SN (2010) The genetic effects of ecological disturbance: flooding in jumping mice. Am Natl 175:126–135
Waples RS (1989) A generalized approach for estimating effective population size from temporal changes in allele frequency. Genetics 121:379–391
Waples RS, Gaggiotti O (2006) What is a population? An empirical evaluation of some genetic methods for identifying the number of gene pools and their degree of connectivity. Mol Ecol 15:1419–1439
Waples RS, Yokota M (2007) Temporal estimates of effective population size in species with overlapping generations. Genetics 175:219–233
Watanabe Y, Dingsør GE, TianY, Tanaka I, Stenseth NC (2008) Determinants of mean length at age of spring spawning herring off the coast of Hokkaido, Japan. Mar Ecol Prog Ser 366:209–217
Wright S (1951) The genetical structure of populations. Ann Eugen 15:323–354
Wright PJ, Galley E, Gibb IM, Neat FC (2006) Fidelity of adult cod to spawning grounds in Scottish waters. Fish Res 77:148–158
Acknowledgements
We thank the editor (Einar Eg Nielsen) and the five anonymous reviewers for their constructive comments, which improved the manuscript. We are grateful to Sho Toriya, and Yukino Ishida for technical assistance and to Akio Iwamoto, Hiroyuki Okouchi, Naoto Murakami, Yoshitomo Nagakura, Takashi Ichikawa, and Daisuke Shimizu for help obtaining samples and providing part of the body measurement data and local catch statistics. This study was supported by a JSPS Grant-in-Aid for Scientific Research (B) 15380133 and 22380110 to SK, and 25280006 and 16H02788 to HK.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflicts of interest.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Kitada, S., Yoshikai, R., Fujita, T. et al. Population structure and persistence of Pacific herring following the Great Tohoku earthquake. Conserv Genet 18, 423–437 (2017). https://doi.org/10.1007/s10592-016-0918-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10592-016-0918-2