Abstract
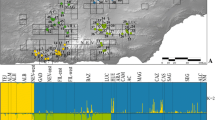
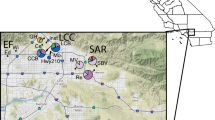
The otton frog (Babina subaspera) is an endangered species endemic to the Amami Islands, Japan. High predation pressure from an introduced carnivore, the mongoose, has caused declines in the frog populations and created a large habitat gap around an urban area. To promote effective conservation, we investigated the genetic status of the species and examined the effect of the habitat gap on gene flow among populations. Using five polymorphic microsatellite loci and mitochondrial DNA sequences, we investigated genetic diversity, genetic structure and gene flow in B. subaspera populations on the islands of Amami-Oshima and Kakeroma-jima. The expected heterozygosity (H E) within each locality was generally high (range: 0.67–0.85), indicating that B. subaspera maintains high genetic diversity. However, genetic differentiation was observed, and the two populations, TAG and KAR, showed little gene flow with other populations. The clustering and F ST analyses also predicted that these two populations were clearly distinct. According to the mitochondrial DNA analysis, the observed genetic differentiation occurred relatively recently. Possible barriers such as mountain ridges, rivers or roads did not result in genetic separation of the populations. These data support the hypothesis that the habitat gap created by an introduced predator prevented the gene flow among B. subaspera populations. When developing conservation strategies for B. subaspera, focus should be directed to these two isolated populations; careful monitoring of population size and genetic diversity should be conducted along with the mongoose elimination project ensues.






Similar content being viewed by others
References
Abe S, Handa Y, Abe Y, Takatsuki Y, Nigi H (1999) Food habits of Feral Mongoose (Herpestes sp.) on Amamioshima, Japan. In: Rodda GH, Sawai Y, Chiszar D, Tanaka H (eds) Problem snake management: the habu and the brown tree snake. Cornel University Press, New York, pp 372–383
Allentoft ME, Siegismund HR, Briggs L, Andersen LW (2009) Microsatellite analysis of the natterjack toad (Bufo calmita) in Denmark: populations are islands in a fragmented landscape. Conserv Genet 10:15–28
Andersen LW, Fog K, Damgaard C (2004) Habitat fragmentation causes bottlenecks and inbreeding in the European tree frog (Hyla arborea). Proc R Soc Lond B 271:1293–1302
Arens P, Bugter R, Westende W, Zollinger R, Stronks J, Vos CC, Smulders MJM (2006) Microsatellite variation and population structure of a recovering tree frog (Hyla arborea L.) metapopulation. Conserv Genet 7:825–834
Blouin MS, Phillipsen IC, Monsen KJ (2010) Population structure and conservation genetics of the oregon spotted frog, Rana pretiosa. Conserv Genet 11:2179–2194
Bohonak AJ (1999) Dispersal, gene flow and population structure. Q Rev Biol 74:21–25
Clement M, Posada D, Cradall KA (2000) TCS: a computer program to estimate gene genealogies. Mol Ecol 9:1657–1660
Cornuet JM, Luikart G (1996) Description and power analysis of two tests for detecting recent population bottlenecks from allele frequency data. Genetics 144:2001–2014
Davis MA (2009) Invasion biology. Oxford University Press, New York
El Mousadik A, Petit RJ (1996) High level of genetic differentiation for allelic richness among populations of the argan tree [Argania spinosa (L) Skeels] endemic to Morocco. Theor Appl Genet 92:832–839
Environment Agency (2000) Threatened wildlife of Japan—red data book, 2nd edn. Japan Wildlife Research Center, Tokyo
Evvano G, Regnaut S, Goudet J (2005) Detecting the number of clusters of individuals using the software STRUCTURE: a simulation study. Mol Ecol 14:2611–2620
Frankham R (1995) Inbreeding and extinction: a threshold effect. Conserv Biol 9:792–799
Frankham R (1997) Do island populations have less genetic variation than mainland populations? Heredity 78:311–327
Frankham R (1998) Inbreeding and extinction: island populations. Conserv Biol 12:665–675
Funk WC, Blouin MS, Corn PS, Maxell BA, Pilliod DS, Amish S, Allendorf FW (2005) Population structure of Columbia spotted frogs (Rana luteiventris) is strongly affected by the landscape. Mol Ecol 14:483–496
Gasc A, Durye MC, Cox RM, Kern A, Calsbeek R (2010) Invasive predators deplete genetic diversity of island lizards. PLoS ONE 5(8):e12061
Goudet J (1995) FSTAT (ver 1.2): a computer program to calculate F-statistics. J Hered 86:485–486
Hubisz M, Falush D, Stephens M, Pritchard J (2009) Inferring weak population structure with the assistance of sample group information. Mol Ecol Resour 9:1322–1332
IUCN (2011) IUCN red list of threatened species. Version 2011.1. http://www.iucnredlist.org. Accessed 10 Aug 2011
Iwai N, Watari Y (2006) Distribution of Ishikawa’s frog and the otton frog on Amami Island. Bull Herpetol Soc Jpn 2:109–114
Iwai N, Shoda-Kagaya E, Hamaguchi K (2011) Isolation and characterization of eight microsatellite loci in the Otton frog, Babina subaspera. J For Res-Jpn. doi:10.1007/s10310-011-0310-5
Kizaki K, Oshiro I (1980) The origin of the Ryukyu islands. In: Kizaki K (ed) Natural history of Ryukyus. Tsukiji-Shokan, Tokyo, pp 8–37
Lesbarrères D, Primmer CR, Lodé T, Merilä J (2006) The effects of 20 years of highway presence on the genetic structure of Rana dalmatina populations. Ecoscience 13:531–538
Lewis DS, vanVeen R, Wildon BS (2011) Conservation implications of small Indian mongoose (Herpestes auropunctatus) predation in a hotspot within a hotspot: the Hellshire Hills, Jamaica. Biol Invasions 13:25–33
Maeda N, Matsui M (1999) Frogs and toads of Japan. Revised ed. Bun-Ichi Sogo Shuppan, Tokyo
Mantel N (1967) The detection of disease clustering and a generalized regression approach. Cancer Res 27:209–220
Matsui M (1996) Natural history of the amphibia. University of Tokyo Press, Tokyo
Ministry of the Environment (2005) Annual report on eradication of introduced Javan mongoose on Amami-Oshima Island. Natural Environment Bureau, Ministry of the Environment, Tokyo
Nei M (1987) Molecular evolutionary genetics. Columbia University Press, New York
Newman D, Pilson D (1997) Increased probability of extinction due to decreased genetic effective population size: experimental populations of Clarkia pulchell. Evolution 51:354–362
Ohbayashi T, Okochi I, Sato H, Ono T, Chiba S (2007) Rapid decline of endemic snails in the Ogasawara Islands, Western Pacific Ocean. Appl Entomol Zool 42:479–485
Oosterhout CV, Hutchinson WF, Wills DPM, Shipley P (2004) MICRO-CHECKER: software for identifying and correcting genotyping errors in microsatellite data. Mol Ecol Notes 4:535–538
Peakall R, Smouse PE (2006) GENALEX 6: genetic analysis in excel. Population genetic software for teaching and research. Mol Ecol Notes 6:288–295
Pritchard JK, Stephens M, Donnelly P (2000) Inference of population structure using multilocus genotype data. Genetics 155:945–959
Raymond M, Rousset F (1995) GENEPOP (version 1.2): population genetics software for exact tests and ecumenicism. J Hered 86:248–249
Rice WR (1989) Analyzing tables of statistical tests. Evolution 43:223–225
Rozas J, Sanchez-DelBarrio JC, Messeguer X, Rozas R (2003) DnaSP, DNA polymorphism analyses by the coalescent and other methods. Bioinformatics 19:2496–2497
Salo P, Korpimäki E, Banks PB, Nordström M, Dickman CR (2007) Alien predators are more dangerous than native predators to prey populations. Proc R Soc B 274:1237–1243
Simões PI, Lima AP, Magnusson WE (2008) Acoustic and morphological differentiation in the frog Allobates femoralis: relationships with the upper Madeira River and other potential geological barriers. Biotropica 40:607–614
Smith FDM, May RM, Pellew R, Johnson TH, Walter KR (1993) How much do we know about the current extinction rate? Trends Ecol Evol 8:375–378
St Clair JJH, Poncet S, Sheehan DK, Szekely T, Hilton GM (2011) Responses of an island endemic invertebrate to rodent invasion and eradication. Anim Conserv 14:66–73
Templeton AR, Crandall KA, Sing CF (1992) A cladistic analysis of phenotypic associations with haplotypes inferred from restriction endonuclease mapping and DNA sequence data III. Cladogram estimation. Genetics 132:619–633
Thompson JD, Gibson TJ, Plewniak F, Jeanmougin F, Higgins DG (1997) The CLUSTAL X windows interface: flexible strategies for multiple sequence alignment aided by quality analysis tools. Nucleic Acids Res 25:4876–4882
Tokita M, Iwai N (2010) Development of the pseudothumb in frogs. Biol Lett 6:517–520
Vences M, Thomas M, Bonett RM, Vieites DR (2005) Deciphering amphibian diversity through DNA barcoding: chances and challenges. Philos Trans R Soc B 360:1859–1868
Vitousek PM (1988) Diversity and biological invasions of oceanic islands. In: Wilson EO, Peters FM (eds) Biodiversity. National Academy Press, Washington, DC, pp 181–189
Watari Y, Takatsuki S, Miyashita T (2008) Effects of exotic mongoose (Herpestes javanicus) on the native fauna of Amami-Oshima Island, southern Japan, estimated by distribution patterns along the historical gradient of mongoose invasion. Biol Invasions 10:7–17
Weir BS, Cockerham CC (1984) Estimating F-statistics for the analysis of population structure. Evolution 38:1358–1370
Wright S (1943) Isolation by distance. Genetics 28:114–138
Acknowledgments
We would like to thank Setsuko Suzuki and Noe Matsushima for their advice. We also thank Yuya Watari and Saya Uemura for their help with field collection. Samples were collected under permits nos. 204 and 703 from the Kagoshima education commission. NI was supported by a Japan Society for the Promotion of Science Research Fellowship and Research Fund.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Iwai, N., Shoda-Kagaya, E. Population structure of an endangered frog (Babina subaspera) endemic to the Amami Islands: possible impacts of invasive predators on gene flow. Conserv Genet 13, 717–725 (2012). https://doi.org/10.1007/s10592-012-0320-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10592-012-0320-7