Abstract
We examined the distribution patterns of native animals on Amami-Oshima Island, southern Japan, along a historical gradient of mongoose establishment and estimated the effects of mongoose on the native fauna. To assess the relative abundance of various ground-dwelling animals, we used the following four methods; sensor cameras for exotic mammals, nighttime driving census for nocturnal native vertebrates, line census for ground-dwelling lizards, and adhesive traps for arthropods. The results indicated that seven species with larger body size, including mammals, birds, reptiles, and amphibians, were rarely observed in mongoose-infested area. By contrast, medium-sized animals showed neutral relationships with mongoose establishment. Interestingly, the densities of smaller-sized animals were higher in mongoose-infested area. It could be interpreted that smaller species have increased in abundance through top-down cascades, i.e., decreases in native predators such as frogs and lizards caused by the mongoose have resulted in increases in the abundance of smaller animals. Predation pressures by mongoose and native predators may be canceled out for medium-sized animals, causing neutral responses to mongoose by these animals. This study appears to be the first example that shows the influence of mongoose on a wide variety of native animals. In addition, our findings indicate the importance of considering the food web structure of a recipient ecosystem and contribute to the prediction and assessment of ecological risks caused not only by mongoose, but also by other invasive top predators.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Invasive species are widely recognized as a major cause of recent biodiversity loss (Vitousek et al. 1997; Wilcove et al. 1998; Mack et al. 2000), and are thought to be responsible for more than 20% of the recent extinctions of vertebrate species (Reid and Miller 1989). Devastation of island faunas by predatory vertebrates has been instrumental in raising awareness of the global threat to biodiversity caused by biological invasion (Elton 1958).
Even a single species of invasive top predator could exert community-wide top–down effects on islands through direct and indirect interactions (Fritts and Rodda 1998), although evidence for such events is scanty. The introduction of the small Indian mongoose (Herpestes javanicus; hereafter, mongoose) to islands within a “biodiversity hotspot area” (Myers et al. 2000) is a good example of the devastating effects that an exotic predator can have on an insular ecosystem (Courchamp et al. 2003). This mongoose, which is native to the area from the Middle East to the Malaya peninsula (e.g. Long 2003), was introduced to many tropical areas, such as the Fijian Islands, Hawaiian Islands, Mauritius, and the West Indies, in the late 1800s to control rats or poisonous snakes (e.g. Long 2003). However, because the mongoose has generalist feeding habits, it also preys on non-target, native species (Pimentel 1955; Gorman 1975; Cavallini and Serafini 1995; Vilella 1998; Abe et al. 1999), and it is now largely blamed for the historical declines and extirpations of many native species on islands (Gorman 1975; Roots 1976; Honegger 1981; Nellis and Small 1983; Nellis et al. 1984; Cheke 1987; Case and Bolger 1991; Henderson 1992). However, few studies have explicitly demonstrated the causal linkage between mongoose invasion and native species degeneration for the following reasons. Firstly, on most of the islands infested by the mongoose, biological information from before the mongoose invasion is lacking, so the cause of decline and extirpation is anecdotal. Secondly, native species that are supposed to have been threatened or extirpated by the mongoose may have been affected synergistically by other invaders or by humans, making it difficult to separate the effects of the mongoose from other factors.
In 1979, 30 mongoose were introduced to Amami-Oshima Island (hereafter Amami Island), southwestern Japan, to control a native poisonous pit viper, habu (Trimeresurus flavoviridis), which was a threat to local people (Tomari 1987; Sawai et al. 1999). Although the mongoose’s ability to control habu is equivocal, mongoose is now established in forest that harbors a number of endemic species and subspecies and the population in 1999 was estimated to be 5,000–10,000 (Ishii 2003). Several studies have revealed that mongoose depends primarily on arthropod prey and also preys on most rare vertebrates (Abe et al. 1999; Environmental Agency et al. 2000; Yamada et al. 2000). This indicates the potential impact of mongoose on many native species. Japan Ministry of the Environment, thus, has begun an eradication project since 2000. However, there are no quantitative assessments of native species, except for a long-term study on the Amami rabbit (Pentalagus furnessi), which shows a decline in its distribution concurrent with the expansion of the mongoose distribution (Sugimura et al. 2000; Yamada et al. 2000).
Fortunately, the mongoose has not spread throughout the island, and there is a spatial gradation of mongoose invasion (Environmental Agency et al. 2000; Ishii 2003; Ministry of the Environment 2005; Fig. 1), which allows us to obtain information along the gradation of the strength of mongoose’s effects. In addition, the ecosystem of Amami Island has suffered fewer disturbances from human activities and other invasive species compared to other mongoose-infested islands. For example, 85% of the land area of Amami Island is still covered by subtropical forest (Sugimura et al. 2003). Invasive mammalian herbivores such as feral pigs or goats, which could alter vegetation, are not present, nor are cats and dogs confirmed to be established (i.e., reproduced) in the forested area. Only the exotic black rat (Rattus rattus) has become established successfully in the forested area. Although many reports have shown the detrimental effects of black rats on insular fauna (e.g. Atkinson 1985; Courchamp et al. 2003), its effects on the native animals on Amami Island is apparently limited, presumably because native species have evolved in the presence of two native rat species, the long-haired rat (Diprothrix legata) and the Amami spinous rat (Tokudaia osimensis). Hence, it appears that Amami Island is an ecosystem that will allow us to tease out the effects of the mongoose alone.
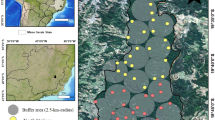
Location of the Amami-Oshima Island, expansion of the mongoose distribution (dotted line) (Environmental Agency et al. 2000; Ministry of the Environment 2005), density of the mongoose (capture/100 TN) estimated by trapping conducted from 1997 to 1999 (grey gradation) (Ishii 2003), and the Amami Central Forest Road (ACF-Road) (Solid line). TN is an abbreviation of the Trap Night. The asterisk represents the release point of the mongoose
To do this, we examined the distribution patterns of native animals on Amami Island along a historical gradient of mongoose establishment and estimated the effects of mongoose on the native fauna. In particular, we focused on ground-dwelling animals as those likely to be affected by mongoose because the mongoose is a poor climber and its diet is composed mainly of ground-dwelling animals (Gorman 1975; Abe et al. 1999). We also investigated what traits of native animals were associated with the differential vulnerability to mongoose and explored the process causing the difference in the vulnerability.
Study area
Amami Island (28°20′ N, 129°14′ E) is the second largest island in the Nansei Islands of Japan, with an area of 712 km2. The forest is dominated by evergreen broadleaved trees such as Catanopsis sieboldii and Schima wallichii, with a steep mountainous topography. Its climate is subtropical, with the average annual temperature and rainfall of 21.5°C and 2,914 mm, respectively.
High priority is given to the conservation of the Nansei Islands because of their high levels of endemism. The World Wide Fund for Nature (WWF) International ranks the forest of Nansei Islands as one of the world’s critical or endangered terrestrial “ecoregions” (http://www.panda.org/about_wwf/where_we_work/ecoregions/ecoregion_list/index.cfm). The forest of Amami Island harbors a large number of the endemic species of the Nansei Islands.
Materials and methods
Study site
Our surveys were conducted along the Amami Central Forest Road (hereafter ACF-Road, 41.1 km long), which begins close to the original release point of the mongoose and leads to areas where the mongoose has not yet become established (Environmental Agency et al. 2000; Ministry of the Environment 2005; Fig. 1). Rather than using snapshot density of the mongoose, we used the distance from the release point as an index of the strength of its effect (hereafter DISTANCE) because the DISTANCE is expected to be negatively correlated with the cumulative density of the mongoose.
Assessing animals
To assess the relative abundance of various ground-dwelling animals, we used the following four methods; sensor cameras for exotic mammals, nighttime driving census for nocturnal native vertebrates, line census for ground-dwelling lizards, and adhesive traps for arthropods. These surveys were conducted from May to October, when most animals were active (Table 1).
A sensor camera (Marif Co., Ltd., Field Note) was set at each of the 27 plots that were established on the forest floor 20–80 m from ACF-Road. The distance between adjacent plots was 1.5 km (Table 1). Bait was placed around the camera, including eggs, fish sausages, dried fishes, sweet potatoes, and peanuts. We checked the cameras and bait every 2–7 days, and changed film, batteries, and bait, if necessary. This survey was conducted for 1 month.
Nighttime driving censuses were started more than 1 h after sunset. We searched for vertebrates occurring on or around the road from a car at a constant speed of about 10 km h−1. We recorded species and location when we encountered vertebrates. We also recorded the call of the Amami rabbit, which is like a vocalization of the pikas (Ochotona) (Yamada and Fernando 2005) and distinguishable from those of other animals. These surveys were conducted four times. Data were converted to presence/absence data per 1.5 km. Small native frogs (<50 mm in snout-vent length) such as the Ryukyu brown frog (Rana okinavana), ornate narrow-mouthed toad (Microhyla ornata), and Ryukyu kajika frog (Buergeria japonica), which were difficult to identify from a car were recorded simply as “small frogs”. Nighttime driving censuses focusing on smaller frogs were conducted on separate days because it was difficult to count small frogs and other animals at the same time. The frequency of occurrence per 1.5 km in the ACF-Road was calculated.
For line censuses, we established 10 additional study plots in the forest along the ACF-Road (Fig. 1, Table 1). The distance between adjacent plots was 1.6–4.6 km. Each plot consisted of a 360–1000-m line along a ridge with a gentle slope. We walked along the line at a constant speed and counted ground-dwelling lizards. The frequency of occurrence of each lizard species per 100 m was calculated for each plot.
To assess arthropods, two adhesive traps (Earth Chemical Co., Ltd., Gokiburi-Hoihoi) were placed on the ground at each of the 27 plots where sensor cameras were established (Table 1). To attract animals, we placed bait inside the traps. We checked the traps every 2–7 days and replaced them if necessary. These surveys were conducted for 1 month. Captured arthropods were counted and the capture rate of each species (individuals trap−1 day−1) was calculated.
Relationships between differential vulnerability and species’ traits
To explore species’ traits that likely to be associated with the vulnerability to mongoose invasion, we examined the relationships between the distribution patterns of native animals and traits of each native animal, i.e., body weight and microhabitat in daytime. The microhabitat in daytime was categorized as “hide” and “exposed”. We hypothesized that these categories may influence encounter rate or detection by the mongoose because mongoose is a diurnal hunter (Long 2003) while all native animals are mostly nocturnal (Watari personal observation). “Hide” means those animals using refuges in daytime such as tree cavity and underneath of dead logs, so that mongoose needs to search actively to detect them. “Exposed” means those animals staying on the ground or trees, or underneath of leaf litter. It seems that mongoose can easily find exposed animals because they flush or come out of litter as mongoose walks around. Traits of native animals used in this study were shown in Appendix A.
Assessing environmental conditions
To confirm that the environmental conditions of the study plots were not correlated with DISTANCE, three environmental properties were measured at each of the 27 plots where sensor cameras were established: steepness of the study plot, basal area at breast height of canopy trees, and amount of leaf litter. In addition, road conditions were surveyed over the whole range of the ACF-Road (Table 1). The amount of leaf litter was measured as the average dry weight of leaf litter (dried at 70°C for 48 h) collected from 10 30 × 30-cm subplots randomly located within each plot. The basal area at breast height was calculated as the total diameter at breast height (DBH) in a 30 × 2-m transect within each plot. The road conditions were categorized as “paved” and “unpaved”.
Statistical methods
To investigate the relationships between the history of mongoose establishment and response variables obtained in this field surveys, we used the linear regression analysis for quantitative variables, and logistic regression analysis for binary variables (Table 2).
To examine the relationships between signs of regression coefficient obtained above and traits of each animal, we used Spearman’s correlation coefficient for body weight and Mann–Whitney’s U test for microhabitat in daytime.
Results
Records of animals
During the surveys, sensor cameras took photos of total of 4 exotic mammals. Because individual identification was difficult, we used presence/absence data at each plot. As expected, mongoose occurrence was negatively correlated with DISTANCE (Fig. 2, Table 2). Exotic black rats were found in almost all plots and their pattern of distribution was not correlated with DISTANCE (Fig. 2, Table 2). A similar tendency was found in the number of photos taken (Fig. 2, Table 2). Cats and dogs were mostly found near the release point of the mongoose (Fig. 2, Table 2).
In nighttime driving censuses, we recorded a total of 9 native vertebrates, including 2 mammals, 1 bird, 3 reptiles, and 3 amphibians (Appendix B). The locations at which vertebrates were found are shown, which were recorded in sufficient numbers to analyze their patterns (Fig. 2). The points at which the Amami rabbit, ground-nesting Amami woodcock (Scolopax mira), Ryukyu odd-tooth snake (Dinodon semicarinatum), Amami tip-nosed frog (Rana amamiensis), Otton frog (Rana subaspera), and Ishikawa’s frog (Rana ishikawae) were found were positively correlated with DISTANCE (Fig. 2, Table 2).
The frequency of occurrence of “small frogs” obtained from nighttime driving censuses showed no relationship with DISTANCE (Fig. 2, Table 2).
The frequency of occurrence of the ground-dwelling Ryukyu short-legged skink (Ateuchosaurus pellopleurus) observed during line censuses showed a positive relationship with DISTANCE (Fig. 2, Table 2).
Arthropods captured using adhesive traps are listed in Appendix C. Those that were thought to be attracted by captured animals were excluded (e.g. ants, bees, spiders, harvestmen, and tiger beetles). The frequencies of occurrence of 4 insect species are shown, which were captured in sufficient numbers to analyze their patterns (Fig. 2). Camel crickets (Diestrammena gigas) showed no relationship with DISTANCE (Fig. 2). In contrast, yellow-spotted crickets (Cardiodactylus novaeguineae), Amami forest cockroaches (Episymploce amamiensis), and Satsuma small cockroaches (Margattea satsumana) showed negative relationships with DISTANCE (Fig. 2, Table 2).
In summary, seven species of vertebrates showed significant decrease in occurrence closer to the original release point of the mongoose, small frogs and the camel cricket were distributed irrespective of the mongoose establishment, and three species of insects increased significantly in occurrence closer to the original release point of the mongoose.
Relationships between vulnerability to the mongoose and species’ traits
Body size of native animals was correlated significantly with the vulnerability to the mongoose estimated by the regression coefficient between occurrence and DISTANCE (Spearman rank correlation: rs = 0.893, P < 0.01; Fig. 3), i.e., larger animals appear to be more abundant in the areas where mongoose is absent or has been established for a shorter period, whereas smaller animals show inverse patterns. However, there was no difference in the vulnerability to mongoose between “hide” and “exposed” animals (Mann–Whitney’s U test, P = 0.120).
Relationships between the body weight of native animals and their response to mongoose invasion. Plus, zero, and minus indicate the sign of regression coefficient of animals against the period of mongoose establishment. Numbered circles represent the following animals: 1: Amami rabbit, 2: Amami woodcock, 3: Ryukyu odd-tooth snake, 4: Otton frog, 5: Ishikawa’s frog, 6: Amami tip-nosed frog, 7: Ryukyu short-legged skink, 8: small frogs, 9: camel cricket, 10: yellow-spotted cricket, 11: Amami forest cockroach, 12: Satsuma small cockroach
Environmental conditions
Regression analyses indicated that none of the four environmental parameters were related to DISTANCE (steepness of plots: linear regression coefficient = 0.285, P = 0.089; basal area at breast height: linear regression coefficient = 0.001, P = 0.851; amount of litter: linear regression coefficient = −0.169, P = 0.115; road conditions: logistic regression coefficient = −0.021, P = 0.57; Fig. 4).
Environmental conditions along the Amami Central Forest Road (ACF-Road) measured in surveys. DISTANCE is the distance from the release point of mongoose along the ACF-Road. (a) Steepness of plots (°), (b) amount of leaf litter (g/30 × 30-cm quadrat), (c) basal area at breast height (m2/30 × 2-m transect), (d) road condition (paved or unpaved)
Discussions
Our results suggest that the mongoose has had a strong negative effect on seven species of ground-dwelling larger native animals, including mammals (Amami rabbit), birds (Amami woodcock), reptiles (Ryukyu odd-tooth snake and Ryukyu short-legged skink), and amphibians (Amami tip-nosed frog, Otton frog, and Ishikawa’s frog), to the extent that they were rarely observed in mongoose-infested areas. All of these are endemic to the Nansei Islands and depend on the forest of Amami Island as the main habitat. Thus, to protect the remaining native animals, it is essential to prevent further expansion of the mongoose’s distribution.
It is at the same time noteworthy that not all ground-dwelling animals were vulnerable to mongoose invasion. In contrast to the larger seven species mentioned above, neutral or even positive relationships with the history of mongoose establishment were shown in middle-sized and smaller-sized animals, respectively. These patterns could not be explained by the direct effects of the mongoose. One possible explanation is that smaller species have increased in abundance through top–down cascades, i.e., decreases in native predators such as frogs, snakes, and lizards caused by the mongoose have resulted in increases in the abundance of smaller animals. This process could be explained by size-selective predation and differential diet ranges between the mongoose and native predators. Although the diet range of the mongoose includes all animals described in this study (Gorman 1975; Abe et al. 1999), larger animals are more likely to be vulnerable to mongoose predation because generalist predators should feed selectively on more profitable prey. On the other hand, prey of frogs, snakes, and lizards, is probably restricted to smaller prey because native predators are smaller than the mongoose and their prey size also appears to be limited by their gape size. Therefore, predation pressure on smaller animals is likely to be stronger from native predators than from the mongoose, leading to a trophic cascade caused by mongoose. Predation pressures by mongoose and native predators may be canceled out for medium-sized animals such as small frogs and the camel cricket, causing neutral responses to mongoose by these animals. As other unknown processes might have been associated with these patterns, further investigations including manipulative field experiments are required.
Our results showed that the effect of microhabitat in daytime of native animals could not explain their distribution patterns. Previous studies showed that prey of the mongoose consist mainly of nocturnal species (Abe et al. 1999; Environmental Agency et al. 2000; Yamada et al. 2000), many of which use refuge in daytime, such as tree cavity or underneath of dead logs. This indicates that the mongoose does search such refuges for prey. Indeed, it was reported that the nest cavity of Amami rabbit was attacked by the mongoose (Yamada personal communication).
Because our study has no replication, the above patterns may have resulted from factors other than the mongoose. However, we have several reasons to believe mongoose causality. Firstly, none of the environmental conditions examined were correlated with the history of mongoose establishment (Fig. 4). Secondly, there are before-invasion records of the Amami rabbit (Sugimura et al. 2000, 2003; Yamada et al. 2000; Sugimura and Yamada 2004) and three species of larger frogs, i.e., the Amami tip-nosed frog, Otton frog, and Ishikawa’s frog (Toyama et al. 1989), in areas where the mongoose has now been established for a long period. Thirdly, the patterns found in this study do not seem to be restricted to our census route. Actually, several target species including Amami rabbit (Sugimura and Yamada 2004), Amami woodcock (Ishida et al. 2003), Otton frog and Ishikawa’s frog (Iwai and Watari 2006), were less abundant in sites other than ACF-Road where the mongoose has now been established for a long period. Lastly, the distribution of other exotics cannot sufficiently explain the detected patterns. The exotic black rat is distributed widely irrespective of the mongoose establishment (Fig. 2). Also, the distributions of cats and dogs were highly biased to areas near the release point of the mongoose, which were clearly more restricted compared with the areas where native vertebrates were absent (Fig. 2).
Unlike the vegetation degeneration or soil erosion caused by large herbivores, community-wide top–down effects induced by exotic top predators are inconspicuous. Moreover, most of the studies assessing the effects of mongoose thus far have been limited to a particular species or taxon. This study appears to be the first example that shows the influence of mongoose on a wide variety of native animals. We also found differential vulnerability among the native species, including positive indirect effects on small animals. This indicates the importance of considering the food web structure of a recipient ecosystem. Our findings contribute to the prediction and assessment of ecological risks caused not only by mongoose, but also by other invasive top predators.
Abbreviations
- ACF Road:
-
Amami Central Forest Road
- Amami Island:
-
Amami-Oshima Island
- DISTANCE:
-
Distance from the release point of the mongoose along the Amami central forest road
References
Abe S, Handa Y, Abe Y, Takatsuki Y, Nigi H (1999) Food habits of Feral Mongoose (Herpestes sp.) on Amamioshima, Japan. In: Rodda GH, Sawai Y, Chiszar D, Tanaka H (eds) Problem snake management: the habu and the brown tree snake. Cornel University Press, New York, pp 372–383
Atkinson IAE (1985) The spread of commensal species of Rattus to oceanic islands and their effect on island avefaunas. In: Moors PJ (ed) Conservation of island birds. ICBP Technical Publication, Cambridge, pp 35–81
Case TJ, Bolger DT (1991) The role of introduced species in shaping the distribution and abundance of island reptiles. Evol Ecol 5:272–290
Cavallini P, Serafini P (1995) Winter diet of the small Indian mongoose, Herpestes auropunctatus, on an Adriatic Island. J Mammal 76:569–574
Cheke AS (1987) An ecological history of the Mascarene Islands, with particular reference to extinctions and introductions of land vertebrates. In: Diamond AW (ed) Studies of Mascarene Island birds. Cambridge University Press, Cambridge, pp 5–89
Courchamp F, Chapuis J-L, Pascal M (2003) Mammal invaders on islands: impact, control and control impact. Biol Rev 78:347–383
Elton CS (1958) The ecology of invasions by animals and plants. Chapman & Hall, London
Environmental Agency, Kagoshima prefecture, Japan Wildlife Research Center (2000) Report on the model project of control of the introduced mongoose on Amami-Oshima Island. Environmental Agency, Kagoshima prefecture, Japan Wildlife Research Center, Tokyo (In Japanese)
Fritts TH, Rodda GH (1998) The role of introduced species in the degradation of island ecosystems: a case history of Guam. Ann Rev Ecol Syst 29:113–140
Gorman ML (1975) The diet of feral Herpestes auropunctatus (Carnivora: Viverridae) in the Fijian Islands. J Zool 175:273–278
Henderson RW (1992) Consequences of predator introductions and habitat destruction on amphibians and reptiles in the post-Columbus West Indies. Carib J Sci 28:1–10
Honegger RE (1981) List of amphibians and reptiles either known or thought to have become extinct since 1600. Biol Conserv 19:141–158
Ishida K, Takashi M, Saito T et al (2003) Ten years’ changes in population and distribution of Amami Wood cocks. Strix 21:99–109 (In Japanese with English summary)
Ishii (2003) Controlling mongooses introduced to Amami-Oshima Island: a population estimate and program evaluation. Jpn J Conserv Ecol 8:73–82 (In Japanese with English summary)
Iwai N, Watari Y (2006) Distribution of Ishikawa’s frog and Otton frog in Amami-Oshima Island. Bull Herpet Soc Jpn 2006(2):109–114. (In Japanese)
Long JL (2003) Introduced mammals of the world: their history, distribution and influence. CABI Publishing, Wallingford
Mack RN, Simberloff D, Lonsdale WM et al (2000) Biotic invasions: causes, epidemiology, global consequences, and control. Ecol Appl 10:689–710
Ministry of the Environment (2005) Annual report on eradication of introduced Javan mongoose on Amami-Oshima Island. Natural Environment Bureau, Ministry of the Environment, Tokyo (In Japanese)
Myers N, Mittermeier RA, Mittermeier CG et al (2000) Biodiversity hotspots for conservation priorities. Nature 403:853–858
Nellis DW, Dewey RA, Hewitt MA et al (1984) Population status of zenaida doves and other Columbids in the Virgin Islands. J Wildl Manag 48:889–894
Nellis DW, Small V (1983) Mongoose predation on sea turtle eggs and nests. Biotropica 15:159–160
Pimentel D (1955) Biology of the Indian mongoose in Puerto Rico. J Mammal 36:62–68
Reid VM, Miller KR (1989) Keeping options alive: the scientific basis for conserving biodiversity. World Resources Institute, Washington, DC
Roots C (1976) Animal invaders. Universe Books, New York
Sawai Y, Kawamura Y, Araki Y et al (1999) A historical outlook on studies of Habu (Trimeresurus flavoviridis) bites in the Amami and Okinawa Islands of Japan. In: Rodda GH, Sawai Y, Chiszar D, Tanaka H (eds) Problem snake management: the habu and the brown tree snake. Cornel University Press, New York, pp 107–115
Sugimura K, Sato S, Yamada F et al (2000) Distribution and abundance of the Amami rabbit Pentalagus furnessi in the Amami and Tokuno Islands, Japan. Oryx 34:198–206
Sugimura K, Yamada F (2004) Estimating population size of the Amami rabbit Pentalagus furnessi based on fecal pellet counts on Amami Island, Japan. Acta Zool Sin 50:519–526
Sugimura K, Yamada F, Miyamoto A (2003) Population trend, habitat change and conservation of the unique wildlife species on Amami Island, Japan. Glob Environ Res 7:79–89
Tomari T (1987) An epidemiologic-study of the occurrence of habu snake bite on the Amami Islands, Japan. Int J Epidem 16:451–461
Toyama M, Kuramoto M, Morita C et al (1989) Distribution of Amphibians and Reptiles within Amami-Oshima Island. In: Natural Environment Bureau, Environmental Agency (ed) Study of essential factors for preservation of wildlife in Nansei Islands. Natural Environment Bureau, Environmental Agency Tokyo, pp 163–171 (In Japanese)
Vilella FJ (1998) Biology of the mongoose (Herpestes javanicus) in a rain forest of Puerto Rico. Biotropica 30:120–125
Vitousek PM, Mooney HA, Lubchenco J et al (1997) Human domination of Earth’s ecosystems. Science 277:494–499
Wilcove DS, Rothstein D, Dubow J et al (1998) Quantifying threats to imperiled species in the United States. Bioscience 48:607–615
World Wide Fund for Nature (2006) List of Ecoregions. http://www.panda.org/about_wwf/where_we_work/ecoregions/ecoregion_list/index.cfm. Cited 28 Aug 2006
Yamada F, Fernando AC (2005) Pentalagus furnessi. Mamm Species 782:1–5
Yamada F, Sugimura K, Abe S et al (2000) Present status and conservation of the endangered Amami rabbit Pentalagus furnessi. Tropics 10:87–92
Acknowledgements
We are especially grateful to Fumio Yamada, Shintaro Abe, Yasunori Maezono, Ryo Yamashita, Ken Ishida, Takehiko Yamanaka, Kazumi Kotaka, Mayura Takada, and Yuki G. Baba for providing us constructive comments and supporting our research. We also thank members of Amami Mammalogical Society and students who helped with our field works. This research was supported by WWF-Nikko Green Investors Fund (FY 2001), WWF Japan Fund Grant Program (FY 2003), and Global Environment Research Fund by Ministry of the Environment Japan ‘F-3’.
Author information
Authors and Affiliations
Corresponding author
Appendix
Appendix
Rights and permissions
Open Access This is an open access article distributed under the terms of the Creative Commons Attribution Noncommercial License ( https://creativecommons.org/licenses/by-nc/2.0 ), which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
About this article
Cite this article
Watari, Y., Takatsuki, S. & Miyashita, T. Effects of exotic mongoose (Herpestes javanicus) on the native fauna of Amami-Oshima Island, southern Japan, estimated by distribution patterns along the historical gradient of mongoose invasion. Biol Invasions 10, 7–17 (2008). https://doi.org/10.1007/s10530-007-9100-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10530-007-9100-6