Abstract
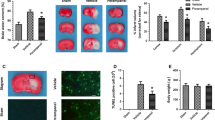
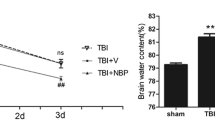
Perampanel is a novel α-amino-3-hydroxy-5-methyl-4-isoxazole propionate receptor (AMPAR) antagonist, approved in over 35 countries as an adjunctive therapy for the treatment of seizures. Recently, it was found to exert protective effects against ischemic neuronal injury in vitro. In the present study, we investigated the potential protective effects of perampanel in a traumatic brain injury (TBI) model in rats. Oral administration with perampanel at a dose of 5 mg/kg exerted no major organ-related toxicities. We found that perampanel significantly attenuated TBI-induced brain edema, brain contusion volume, and gross motor dysfunction. The results of Morris water maze test demonstrated that perampanel treatment also improved cognitive function after TBI. These neuroprotective effects were accompanied by reduced neuronal apoptosis, as evidenced by decreased TUNEL-positive cells in brain sections. Moreover, perampanel markedly inhibited lipid peroxidation and obviously preserved the endogenous antioxidant system after TBI. In addition, enzyme-linked immunosorbent assay (ELISA) was performed at 4 and 24 h after TBI to evaluate the expression of inflammatory cytokines. The results showed that perampanel suppressed the expression of pro-inflammatory cytokines TNF-α and IL-1β, whereas increased the levels of anti-inflammatory cytokines IL-10 and TGF-β1. These data show that the orally active AMPAR antagonist perampanel affords protection against TBI-induced neuronal damage and neurological dysfunction through anti-oxidative and anti-inflammatory activity.






Similar content being viewed by others
References
Arundine M, Tymianski M (2004) Molecular mechanisms of glutamate-dependent neurodegeneration in ischemia and traumatic brain injury. Cell Mol Life Sci 61(6):657–668. doi:10.1007/s00018-003-3319-x
Bermpohl D, You Z, Lo EH, Kim HH, Whalen MJ (2007) TNF alpha and Fas mediate tissue damage and functional outcome after traumatic brain injury in mice. J Cereb Blood Flow Metab 27(11):1806–1818. doi:10.1038/sj.jcbfm.9600487
Bolton C, Paul C (2006) Glutamate receptors in neuroinflammatory demyelinating disease. Mediat Inflamm 2:93684. doi:10.1155/MI/2006/93684
Calabresi P, Centonze D, Cupini LM, Costa C, Pisani F, Bernardi G (2003) Ionotropic glutamate receptors: still a target for neuroprotection in brain ischemia? Insights from in vitro studies. Neurobiol Dis 12(1):82–88
Ceolin L, Bortolotto ZA, Bannister N, Collingridge GL, Lodge D, Volianskis A (2012) A novel anti-epileptic agent, perampanel, selectively inhibits AMPA receptor-mediated synaptic transmission in the hippocampus. Neurochem Int 61(4):517–522. doi:10.1016/j.neuint.2012.02.035
Cernak I (2005) Animal models of head trauma. NeuroRx J Am Soc Exp NeuroTher 2(3):410–422. doi:10.1602/neurorx.2.3.410
Chang J, Phelan M, Cummings BJ (2015) A meta-analysis of efficacy in pre-clinical human stem cell therapies for traumatic brain injury. Exp Neurol 273:225–233. doi:10.1016/j.expneurol.2015.08.020
Chao CC, Hu S, Ehrlich L, Peterson PK (1995) Interleukin-1 and tumor necrosis factor-alpha synergistically mediate neurotoxicity: involvement of nitric oxide and of N-methyl-D-aspartate receptors. Brain Behav Immun 9(4):355–365. doi:10.1006/brbi.1995.1033
Chen T, Liu W, Chao X, Zhang L, Qu Y, Huo J, Fei Z (2011) Salvianolic acid B attenuates brain damage and inflammation after traumatic brain injury in mice. Brain Res Bull 84(2):163–168. doi:10.1016/j.brainresbull.2010.11.015
Chua KS, Ng YS, Yap SG, Bok CW (2007) A brief review of traumatic brain injury rehabilitation. Ann Acad Med, Singap 36(1):31–42
Correale J, Villa A (2004) The neuroprotective role of inflammation in nervous system injuries. J Neurol 251(11):1304–1316. doi:10.1007/s00415-004-0649-z
Davis AE (2000) Cognitive impairments following traumatic brain injury. Etiologies and interventions. Crit Care Nurs Clin North Am 12(4):447–456
Dixon CE, Clifton GL, Lighthall JW, Yaghmai AA, Hayes RL (1991) A controlled cortical impact model of traumatic brain injury in the rat. J Neurosci Methods 39(3):253–262
Durand D, Pampillo M, Caruso C, Lasaga M (2008) Role of metabotropic glutamate receptors in the control of neuroendocrine function. Neuropharmacology 55(4):577–583. doi:10.1016/j.neuropharm.2008.06.022
Frenkel D, Huang Z, Maron R, Koldzic DN, Hancock WW, Moskowitz MA, Weiner HL (2003) Nasal vaccination with myelin oligodendrocyte glycoprotein reduces stroke size by inducing IL-10-producing CD4+ T cells. J Immunol 171(12):6549–6555
Grossman SA, Ye X, Chamberlain M, Mikkelsen T, Batchelor T, Desideri S, Piantadosi S, Fisher J, Fine HA (2009) Talampanel with standard radiation and temozolomide in patients with newly diagnosed glioblastoma: a multicenter phase II trial. J Clin Oncol 27(25):4155–4161. doi:10.1200/JCO.2008.21.6895
Hanada T, Hashizume Y, Tokuhara N, Takenaka O, Kohmura N, Ogasawara A, Hatakeyama S, Ohgoh M, Ueno M, Nishizawa Y (2011) Perampanel: a novel, orally active, noncompetitive AMPA-receptor antagonist that reduces seizure activity in rodent models of epilepsy. Epilepsia 52(7):1331–1340. doi:10.1111/j.1528-1167.2011.03109.x
Henley JM, Wilkinson KA (2013) AMPA receptor trafficking and the mechanisms underlying synaptic plasticity and cognitive aging. Dialogues Clin Neurosci 15(1):11–27
Hibi S, Ueno K, Nagato S, Kawano K, Ito K, Norimine Y, Takenaka O, Hanada T, Yonaga M (2012) Discovery of 2-(2-oxo-1-phenyl-5-pyridin-2-yl-1,2-dihydropyridin-3-yl)benzonitrile (perampanel): a novel, noncompetitive alpha-amino-3-hydroxy-5-methyl-4-isoxazolepropanoic acid (AMPA) receptor antagonist. J Med Chem 55(23):10584–10600. doi:10.1021/jm301268u
Hwabejire JO, Jin G, Imam AM, Duggan M, Sillesen M, Deperalta D, Jepsen CH, Lu J, Li Y, deMoya MA, Alam HB (2013) Pharmacologic modulation of cerebral metabolic derangement and excitotoxicity in a porcine model of traumatic brain injury and hemorrhagic shock. Surgery 154(2):234–243. doi:10.1016/j.surg.2013.04.008
Israelsson C, Bengtsson H, Kylberg A, Kullander K, Lewen A, Hillered L, Ebendal T (2008) Distinct cellular patterns of upregulated chemokine expression supporting a prominent inflammatory role in traumatic brain injury. J Neurotrauma 25(8):959–974. doi:10.1089/neu.2008.0562
Jiang X, Huang Y, Lin W, Gao D, Fei Z (2013) Protective effects of hydrogen sulfide in a rat model of traumatic brain injury via activation of mitochondrial adenosine triphosphate-sensitive potassium channels and reduction of oxidative stress. J Surg Res 184(2):e27–e35. doi:10.1016/j.jss.2013.03.067
Koeglsperger T, Li S, Brenneis C, Saulnier JL, Mayo L, Carrier Y, Selkoe DJ, Weiner HL (2013) Impaired glutamate recycling and GluN2B-mediated neuronal calcium overload in mice lacking TGF-beta1 in the CNS. Glia 61(6):985–1002. doi:10.1002/glia.22490
Lea PMt, Faden Al (2001) Traumatic brain injury: developmental differences in glutamate receptor response and the impact on treatment. Ment Retard Dev Disabil Res Rev 7(4):235–248. doi:10.1002/mrdd.1033
Lighthall JW, Goshgarian HG, Pinderski CR (1990) Characterization of axonal injury produced by controlled cortical impact. J Neurotrauma 7(2):65–76
Mao H, Zhang L, Yang KH, King AI (2006) Application of a finite element model of the brain to study traumatic brain injury mechanisms in the rat. Stapp Car Crash J 50:583–600
Morganti-Kossmann MC, Rancan M, Otto VI, Stahel PF, Kossmann T (2001) Role of cerebral inflammation after traumatic brain injury: a revisited concept. Shock 16(3):165–177
Nichol A, French C, Little L, Haddad S, Presneill J, Arabi Y, Bailey M, Cooper DJ, Duranteau J, Huet O, Mak A, McArthur C, Pettila V, Skrifvars M, Vallance S, Varma D, Wills J, Bellomo R (2015) Erythropoietin in traumatic brain injury (EPO-TBI): a double-blind randomised controlled trial. Lancet. doi:10.1016/S0140-6736(15)00386-4
Obrenovitch TP, Urenjak J (1997) Is high extracellular glutamate the key to excitotoxicity in traumatic brain injury? J Neurotrauma 14(10):677–698
Olney JW, Labruyere J, Wang G, Wozniak DF, Price MT, Sesma MA (1991) NMDA antagonist neurotoxicity: mechanism and prevention. Science 254(5037):1515–1518
Ooboshi H, Ibayashi S, Shichita T, Kumai Y, Takada J, Ago T, Arakawa S, Sugimori H, Kamouchi M, Kitazono T, Iida M (2005) Postischemic gene transfer of interleukin-10 protects against both focal and global brain ischemia. Circulation 111(7):913–919. doi:10.1161/01.CIR.0000155622.68580.DC
Pang L, Ye W, Che XM, Roessler BJ, Betz AL, Yang GY (2001) Reduction of inflammatory response in the mouse brain with adenoviral-mediated transforming growth factor-ss1 expression. Stroke 32(2):544–552
Schumann J, Alexandrovich GA, Biegon A, Yaka R (2008) Inhibition of NR2B phosphorylation restores alterations in NMDA receptor expression and improves functional recovery following traumatic brain injury in mice. J Neurotrauma 25(8):945–957. doi:10.1089/neu.2008.0521
Seeburg PH, Single F, Kuner T, Higuchi M, Sprengel R (2001) Genetic manipulation of key determinants of ion flow in glutamate receptor channels in the mouse. Brain Res 907(1–2):233–243
Sheardown MJ, Nielsen EO, Hansen AJ, Jacobsen P, Honore T (1990) 2,3-Dihydroxy-6-nitro-7-sulfamoyl-benzo(F)quinoxaline: a neuroprotectant for cerebral ischemia. Science 247(4942):571–574
Simma N, Bose T, Kahlfuss S, Mankiewicz J, Lowinus T, Luhder F, Schuler T, Schraven B, Heine M, Bommhardt U (2014) NMDA-receptor antagonists block B-cell function but foster IL-10 production in BCR/CD40-activated B cells. Cell Commun Signal 12:75. doi:10.1186/s12964-014-0075-5
Smith SE, Meldrum BS (1993) Cerebroprotective effect of a non-N-methyl-D-aspartate antagonist, NBQX, after focal ischaemia in the rat. Funct Neurol 8(1):43–48
Spaethling J, Le L, Meaney DF (2012) NMDA receptor mediated phosphorylation of GluR1 subunits contributes to the appearance of calcium-permeable AMPA receptors after mechanical stretch injury. Neurobiol Dis 46(3):646–654. doi:10.1016/j.nbd.2012.03.003
Stone TW, Addae JI (2002) The pharmacological manipulation of glutamate receptors and neuroprotection. Eur J Pharmacol 447(2–3):285–296
Turetsky D, Garringer E, Patneau DK (2005) Stargazin modulates native AMPA receptor functional properties by two distinct mechanisms. J Neurosci 25(32):7438–7448. doi:10.1523/JNEUROSCI.1108-05.2005
Walters MR, Kaste M, Lees KR, Diener HC, Hommel M, De Keyser J, Steiner H, Versavel M (2005) The AMPA antagonist ZK 200775 in patients with acute ischaemic stroke: a double-blind, multicentre, placebo-controlled safety and tolerability study. Cerebrovasc Dis 20(5):304–309. doi:10.1159/000087929
Weiser T (2005) AMPA receptor antagonists for the treatment of stroke. Curr Drug Targets CNS Neurol Disord 4(2):153–159
Winter CD, Iannotti F, Pringle AK, Trikkas C, Clough GF, Church MK (2002) A microdialysis method for the recovery of IL-1beta, IL-6 and nerve growth factor from human brain in vivo. J Neurosci Methods 119(1):45–50
Woodroofe MN, Sarna GS, Wadhwa M, Hayes GM, Loughlin AJ, Tinker A, Cuzner ML (1991) Detection of interleukin-1 and interleukin-6 in adult rat brain, following mechanical injury, by in vivo microdialysis: evidence of a role for microglia in cytokine production. J Neuroimmunol 33(3):227–236
Xiong Y, Mahmood A, Chopp M (2013) Animal models of traumatic brain injury. Nat Rev Neurosci 14(2):128–142. doi:10.1038/nrn3407
Ziebell JM, Morganti-Kossmann MC (2010) Involvement of pro- and anti-inflammatory cytokines and chemokines in the pathophysiology of traumatic brain injury. Neurotherapeutics 7(1):22–30. doi:10.1016/j.nurt.2009.10.016
Acknowledgments
This work was supported by the National Natural Science Foundation of China (Nos. 81371447 and 81301037).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
There is no conflict of interest.
Additional information
Tao Chen, Shu-Hui Dai and Zhi-Quan Jiang have contributed equally to this work.
Rights and permissions
About this article
Cite this article
Chen, T., Dai, SH., Jiang, ZQ. et al. The AMPAR Antagonist Perampanel Attenuates Traumatic Brain Injury Through Anti-Oxidative and Anti-Inflammatory Activity. Cell Mol Neurobiol 37, 43–52 (2017). https://doi.org/10.1007/s10571-016-0341-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10571-016-0341-8