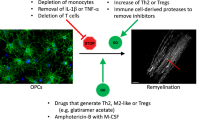
Abstract
The contribution of inflammation to the pathogenesis of several nervous system disorders has long been established. Other observations, however, indicate that both inflammatory cells and mediators may also have beneficial functions, assisting in repair and recovery processes. There is compelling evidence to indicate that in the injured nervous system, as in other tissues, macrophages are needed at an early stage after injury in order for healing to take place. Likewise, activated T cells of a particular specificity can reduce the spread of damage. This neuroprotective effect of T cells may be caused, at least in part, by the production of neurotrophic factors such as neurotrophin-3 or brain-derived neurotrophic factor. Interestingly, recent findings indicate that immune cells are able to produce a variety of neurotrophic factors which promote neuronal survival and may also mediate anti-inflammatory effects. Numerous cytokines are induced after nervous system injuries. Some cytokines, such as TNF-α, IL-1 and IFN-γ, are well known for their promotion of inflammatory responses. However, these cytokines also have immunosuppressive functions and their subsequent expression also assists in repair or recovery processes, suggesting a dual role for some pro-inflammatory cytokines. This should be clarified, as it may be crucial in the design of therapeutic strategies to target specific cytokine(s). Finally, there is a growing body of evidence to show that autoreactive IgM antibodies may constitute an endogenous system of tissue repair, and therefore prove of value as a therapeutic strategy. Available evidence would appear to indicate that the inflammatory response observed in several neurological conditions is more complex than previously thought. Therefore, the design of more effective therapies depends on a clear delineation of the beneficial and detrimental effects of inflammation.
Similar content being viewed by others
References
Albrecht PJ, Dahl JP, Stoltzfus OK, Levenson R, Levinson SW (2002) Ciliary neurotrophic factor activates spinal cord astrocytes, stimulating their production and release of fibroblast growth factor-2, to increase motor neuron survival. Exp Neurol 173:46–62
Alexander WS, Starr R, Fenner JE, Scott CL, Handmann E, Sprigg NS, Corbin JE, Cornish AL, Darwiche R, Owczareck CM, Kay TW, Nicola NA, Hertzog PJ, Metcalf D, Hilton DJ (1999) SOCS1 is a critical inhibitor of interferon gamma signalling and prevents the potentially fatal neonatal actions of this cytokine. Cell 98:597–608
Aloisi F, Borsellino G, Samoggia P, Testa U, Chelucci C, Russo G, Peschle C, Levi G (1992). Astrocyte cultures from human embryonic brain: characterization and modulation of surface molecules by inflammatory cytokines. J Neurosci Res 32:494–506
Aloisi F, Ria F, Penna G, Adorini L (1998) Microglia are more efficient than astrocytes in antigen processing and in Th1 but not Th2 cell activation. J Immunol 160:4671–4680
Aloisi F, Penna G, Polazzi E, Minghetti L,Adorini L (1999) CD40-CD154 interaction and IFN-gamma are required for IL-12 but not prostaglandin E2 secretion by microglia during antigen presentation to Th1 cells (1999). J Immunol 162:1384–1391
Antel JP, Becher B (1998) Central nervous system—Immune interactions: Contribution to neurologic disease and recovery. In: Antel J, Birnbaum G, Hartung HP (eds) Clinical Neuroimmunology. Blackwell Science, Oxford, pp 26–39
Antel J, Birnbaum G, Hartung HP (1998) Clinical Neuroimmunology. Blackwell Science, Oxford
Armati PJ, Pollard JD, Gatenby P (1990) Rat and human Schwann cells in vitro can synthesize and express MHC molecules. Muscle Nerve 13:106–116
Arnett HA, Masson J, Marino M, Suzuki K, Matsushima GK, Ting JPY (2001) TNFα promotes proliferation of oligodendrocyte progenitors and remyelination. Nature Neurosci 4:1116–1122
Arnett HA, Wang Y, Matsushima GK, Suzuki K, Ting JPY (2003) Functional genomic analysis of remyelination reveals importance of inflammation in oligodendrocytes regeneration. J Neurosci 23:9824–9832
Asada H, Ip NY, Pan L, Razack N, Parfitt MM, Plunkett RJ (1995) Time course of ciliary neurotrophic factor mRNA expression is coincident with the presence of protoplasmic astrocytes in traumatized rat striatum. J Neurosci Res 40:22–30
Asakura K, Miller DJ, Murray P, Bansal R, Pfeiffer SE, Rodriguez M (1996) Monoclonal autoantibody SCH94.03, which promotes central nervous system remyelination, recognizes an antigen on the surface of oligodendrocytes. J Neurosci Res 43:273–281
Asakura K, Miller DJ, Pease LR, Rodriguez M (1998) Targeting of IgMκ antibodies to oligodendrocytes promotes CNS remyelination. J Neurosci 18:7700–7708
Asakura K, Rodriguez M (1998) A unique population of circulating autoantibodies promotes central nervous system remyelination. Mult Scler 4:217–221
Bai XF, Zhu J, Zhang GX, Kaponides G, Hojeberg B, van der Meide PH, Link H (1997) IL-10 suppresses experimental autoimmune neuritis and downregulates cytokine mRNA expression of Th1 and macrophage source. Clin Immunol Immunopathol 83:117–126
Baloh RH, Enomoto H, Johnson EM Jr, Milbrandt J (2000) The GDNF family ligands and receptors-implications for neural development. Curr Opin Neurobiol 10:103–110
Barde YA, Edgar D, Thoenen H (1983) New neurotrophic factors. Annu Rev Physiol 45:601–612
Barouch R, Appel E, Kazimirsky G, Brodie C (2001) Macrophages express neurotrophins and neurotrophin receptors. Regulation of nitric oxide production by NT-3. J Neuroimmunol 112:72–77
Barouch R, Schwartz M (2002) Autoreactive T cells induce neurotrophin production by immune and neural cells in injured rat optic nerve: Implications for protective autoimmunity. FASEB J 16:1304–1306
Batchelor PE, Liberatore GT, Wong JYF, Porrit MJ, Frerichs F, Donnan GA, Howells DW (1999) Activated macrophages and microglia induce dopaminergic sprouting in the injured striatum and express brain-derived neurotrophic factor and glial cell line-derived neurotrophic factor. J Neurosci 19:1708–1716
Becher B, Antel JP (1996) Comparison of phenotypic and functional properties of immediately ex vivo and cultured human adult microglia. Glia 18:1–10
Be’eri H, Reichert F, Saada A, Rotshenker S (1998) The cytokine network of wallerian degeneration: IL-10 and GM-CSF. Eur J Neurosci 10:2707–2713
Besser M, Wang R (1999) Clonally restricted production of the neurotrophin brain-derived neurotrophic factor and neurotrophin-3 mRNA by human immune cells and Th1/Th2-polarized expression of their receptors. J Immunol 162:6303–6306
Bieber AJ, Warrington A, Asakura K, Ciric B, Kaveri SV, Pease LR, Rodriguez M (2002) Human antibodies accelerate the rate of remyelination following lysolecithin-induced demyelination in mice. Glia 37:241–249
Bieber AJ, Kerr S, Rodriguez M (2003) Efficient central nervous system remyelination requires T cells. Ann Neurol 53:680–684
Billiau A, Heremans H, Vandekerckhove F, Dijkmans R, Sobis H, Meulepas E, Carton H (1988) Enhancement of experimental allergic encephalomyelitis in mice by antibodies against IFN-gamma. J Immunol 140:1506–1510
Burns J, Rosenzweig A, Zweiman B, Lisak RP (1983) Isolation of myelin basic protein-reactive T cell lines from normal human blood. Cell Immunol 81:435–440
Brück W, Huitinga I, Dijkstra CD (1996) Liposome-mediated monocyte depletion during Wallerian degeneration defines the role of hematogenous phagocytes in myelin removal. J Neurosci Res 46:477–484
Chaudhry V, Glass JD, Griffin JW (1992) Wallerian degeneration in peripheral nerve disease. Neurol Clin 10:613–627
Chen MS, Huber AB, van der Haar ME, Frank M, Scnell L, Spillman AA, Christ F, Schwab ME (2000) Nogo-A is a myelin-associated neurite outgrowth inhibitor and an antigen for monoclonal antibody IN-1. Nature 403:434–439
Ciric B, Howe CL, Paz Soldan M, Warrington AE, Bieber AJ, van Keulen V, Rodriguez M, Pease LR (2003) Human monoclonal IgM antibody promotes CNS myelin repair independent of Fc function. Brain Pathol 13:608–616
Ciric B, Van Keulen V, Paz Soldan M, Rodriguez M, Pease LR (2004) Antibody-mediated remyelination operates through mechanism independent of immunomodulation. J Neuroimmunol 146:153–161
Correale J, McMillan M, McCarthy K, Le T, Weiner LP (1995) Isolation and characterization of autoreactive proteolipid protein-peptide specific T-cell clones from multiple sclerosis patients. Neurology 45:1370–1378
Correale J, Bassani Molinas MM (2002) Oligoclonal bands and antibody responses in Multiple Sclerosis. J Neurol 249:375–389
Cowley SA, Butter C, Gschmeissner SE, Curtis J, Turk JL (1989) An immuno-electromicroscopical study of the expression of major histocompatibility complex (MHC) class II antigens in guinea pig sciatic nerves following induction of intraneural mycobacterial granulomas. J Neuroimmunol 23:223–231
Creange A, Barlovatz-Meimon G, Gherardi RK (1997) Cytokines and peripheral nerve disorders. Eur Cytokine Netw 8:145–151
David S, Aguayo AJ (1981) Axonal elongation into peripheral nervous system “bridges” after central nervous system injury in adult rats. Science 214:931–933
David S, Aguayo AJ (1985) Axonal regeneration after crush injury in rat central nervous system fibers innervating peripheral nerve grafts. J Neurocytol 14:1–12
David S, Bouchard C, Tsatas O, Giftochristos N (1990) Macrophages can modify the nonpermissive nature of the adult mammalian central nervous system. Neuron 5:463–469
Day WA, Kosihi K, McLennan IS (2003) Transforming growth factor beta 1 may regulate the stability of mature myelin sheath. Exp Neurol 184:857–864
Deller T, Haas CA, Naumann T, Joester A, Faissner A, Frotscher M (1997) Up-regulation of astrocyte-derived tenascin-C correlates with neurite out-growth in the rat dentate gyrus after unilateral entorhinal cortex lesion. Neuroscience 81:829–846
De Jong BA, Smith ME (1997) A role for complement in phagocytosis of myelin. Neurochem Res 22:491–498
De Kosky ST, Styren SD, O’Malley ME, Gross JR, Kochanek P, Marion D, Evans CH, Robbins PD (1996) Interleukin-1 receptor antagonist suppresses neurotrophin response in injured rat brain. Ann Neurol 39:123–127
Duong TT, St Louis J, Gilbert JJ, Finkelman FD, Strejan GH (1992) Effect of anti-interferon gamma and anti-interleukin-2 monoclonal antibody treatment on the development of actively and passively induced experimental allergic encephalomyelitis in the SJL/J mouse. J Neuroimmunol 36:105–115
Dyer CA, Benjamins JA (1989) Organization of oligodendrocytes membrane sheets. II. Galactocerebroside: antibody interactions signal changes in cytoskeleton and myelin basic protein. J Neurosci Res 24:212–221
Dyer CA, Benjamins JA (1991) Galactocerebroside and sulfatide independently mediate Ca2+ responses in oligodendrocytes. J Neurosci Res 30:699–711
Dyer CA, Matthieu JM (1994) Antibodies to myelin/oligodendrocyte-specific protein and myelin/oligodendrocyte-specific glycoprotein signal distinct changes in the organization of cultured oligodendroglial membranes. J Neurochem 62:777–787
Ehrhard PB, Erb P, Garumann U, Otten U (1993) Expression of nerve growth factor and nerve growth factor receptor tyrosine kinase Trk in activated CD4-positive T cell clones. Proc Natl Acad Sci USA 90:10984–10988
Fernández-Valle C, Bunge RP, Bunge MB (1995) Schwann cells degrade myelin and proliferate in the absence of macrophages: evidence from in vitro studies of Wallerian degeneration. J Neurocytol 24:667–679
Flugel A, Matsumuro K, Neumann H, Klinkert WE, Birnbacher R, Lassmann H, Otten U, Wekerle H (2001) Anti-inflammatory activity of nerve growth factor in experimental autoimmune encephalomyelitis inhibition of monocyte trans-endothelial migration. Eur J Immunol 31:11–22
Fontana A, Fierz F, Wekerle H (1994) Astrocytes present myelin basic protein to encephalitogenic T-cell lines. Nature 307:273–276
Friedman B, Scherer SS, Rudge JS, Helgren M, Morriscy D, McIain J, Wang DY, Wiegand SJ, Furth ME, Lindsay RM (1992) Regulation of ciliary neurotrophic factor expression in myelinrelated Schwann cells in vivo. Neuron 9:295–305
Furlan R, Brambilla E, Ruffini F, Poliani PL, Bergami A, Marconi PC, Franciotta DM, Penna G, Comi G, Adorini L, Martino G (2001) Intrathecal delivery of IFN-gamma protects C57BL/6 mice from chronic-progressive experimental autoimmune encephalomyelitis by increasing apoptosis of central nervous system-infiltrating lymphocytes. J Immunol 167:1821–1829
George R, Griffin JW (1994) Delayed macrophage responses and myelin clearance during wallerian degeneration in the central nervous system: the dorsal radiculotomy model. Exp Neurol 129:225–236
Giulian D, Baker TJ, Shih LC, Lachman LB (1986) Interleukin 1 of the central nervous system is produced by ameboid microglia. J Exp Med 164:594–604
Giulian D, Woodward J, Young DG, Krebs JF, Lachman LB (1988) Interleukin-1 injected into mammalian brain stimulates astrogliosis and neovascularization. J Neurosci 8:2485–2490
Gold T, Toyka KV, Hartung HP (1995) Synergistic effect of IFN-gamma and TNF-alpha on expression of immune molecules and antigen presentation by Schwann cells. Cell Immunol 165:65–70
Griffin JW, Stoll G, Li CY, Tyor W, Cornblath DR (1990) Macrophage responses in demyelinating neuropathies. Ann Neurol 27:S64–S68
Griffin JW, Li CY, Ho TW, Tian M, Gao CY, Xue P, Mishu B, Cornblath DR, Macko C, McKhann GM, Asbury AK (1996) Pathology of the motor-sensory axonal Guillain-Barré. Ann Neurol 39:17–28
Grothe C, Meisinger C, Claus P (2001) In vivo expression and localization of the fibroblast growth factor system in the intact and lesioned rat peripheral nerve and spinal ganglia. J Comp Neurol 434:342–357
Guenard V, Dinarello CA, Weston PJ, Aebischer P (1991) Peripheral nerve regeneration is impeded by interleukin-1 receptor antagonist released from a polymeric guidance channel. J Neurosc Res 29:396–400
Guillen C, Jander S, Stoll G (1998) Sequential expression of mRNA for proinflammatory cytokines and interleukin-10 in the rat peripheral nervous system: comparison between immune-mediated demyelination and Wallerian degeneration. J Neurosci Res 51:489–496
Hagg T, Varon S (1993) Ciliary neurotrophic factor prevents degeneration of adult rats substantia nigra dopaminergic neurons in vivo. Proc Natl Acad Sci 90:6315–6319
Hammarberg H, Lidman O, Lundberg C, Eltayeb SY, Gielen AW, Muhallab S, Svenningsson A, Lindå H, van der Meide PH, Culheim S, Olsson T, Piehl F (2000) Neuroprotection by encephalomyelitis: rescue of mechanically injured neurons and neurotrophin production by CNS-infiltrating T and natural killer cells. J Neurosci 20:5283–5291
Hartung HP, Schafer B, van der Meide PH, Fierz W, Heininger K, Toyka KV (1990) The role of interferon-gamma in the pathogenesis of experimental autoimmune disease of the peripheral nervous system. Ann Neurol 27:247–257
Hattori A, Iwasaki S, Murase K, Tsujimoto M, Sato M, Hayashi K, Khono M (1994) Tumor necrosis factor is markedly synergistic with interleukin 1 and interferon-γ in stimulating the production of nerve growth factor in fibroblasts. FEBS Letters 340:177–180
Hauben E, Nevo U, Yoles E, Moaleem G, Agranov E, Mor F, Akselrod S, Neeman M, Cohen IR, Schwartz M (2000) Autoimmune T cells as potential neuroprotective therapy for spinal cord injury. Lancet 355:286–287
Hauben E, Gothilf A, Cohen A, Butovsky O, Nevo U, Smirnov I, Yoles E, Akselrod S, Schwartz M (2003) Vaccination with dendritic cells pulsed with peptides of myelin basic protein promotes functional recovery from spinal cord injury. J Neurosci 23:8808–8819
Henderson CE, Phillips HS, Pollock RA, Davies Am, Lemeulle C, Armanini M, Simpson LC, MoVet B, Vandlen RA, Koliatsos VE, Rosenthal A (1994) GDNF: a potent survival factor for motoneurons present in peripheral nerve and muscle. Science 266:1062–1064
Herx LM, Rivest S, Yong VW (2000) Central nervous system-initiated inflammation and neurotrophism in trauma: IL-1β is required for the production of ciliary neurotrophic factor. J Immunol 165:2232–2239
Heumann R, Korsching S, Bandtlow C, Thoenen H (1987) Changes of nerve growth factor synthesis in non neuronal cells response to sciatic nerve transection. J Cell Biol 104:1623–1631
Ho A, Blum M (1997) Regulation of astroglial-derived dopaminergic neurotrophic factors by interleukin-1 beta in striatum of young and middle-aged mice. Exp Neurol 148:348–359
Howe CL, Bieber AJ, Warrington AE, Pease LR, Rodriguez M (2004) Anti-apoptotic signalling by a remyelination-promoting human antimyelin antibody. Neurobiol Dis 15:120–131
Ip NY (1998) The neurotrophins and neuropoietic cytokines. Two families of growth factors acting on neural and hematopoietic cells. Ann N Y Acad Sci 840:97–106
Iseda T, Nishio T, Kawaguchi S, Yamanoto M, Kawasaki T, Wakisaka S (2004) Spontaneous regeneration of the corticospinal tract after transsection in young rats: a key role of reactive astrocytes in making favourable and unfavourable conditions for regeneration. Neuroscience 126:365–374
Jung S, Huitinga I, Schmidt B, Zielasek J, Dijkstra CD, Toyka KV, Hartung HP (1993) Selective elimination of macrophages by dichloromethylene diphosphonate-containing liposomes suppresses experimental autoimmune neuritis. J Neurol Sci 119:195–202
Karnezis T, Mandemakers W, McQualter JL, Zheng B, Ho PP, Jordan KA, Murray BM, Barres B, Tessier-Levigne M, Bernard CC (2004) The neurite out-growth inhibitor Nogo A is involved in autoimmune-mediated demyelination. Nature Neurosci 7:736–744
Kassiotis G, Kollias G (2001) Uncoupling the proinflammatory from the immunosuppressive properties of tumor necrosis factor (TNF) at the p55 TNF receptor level: Implications for pathogenesis and therapy of autoimmune demyelination. J Exp Med 193:427–434
Kawaja MD, Gage FH (1991) Reactive astrocytes are substarte for the growth of adult CNS axons in the presence of elevated levels of nerve growth factors. Neuron 7:1019–1030
Kerschensteiner M, Gallmeier E, Behrens L, Vargas Leal V, Misgeld T, Klinkert WEF, Kolbeck R, Hoppe E, Oropeza-Wekerle R, Bartke I, Stadelmann C, Lassmann H, Wekerle H, Hohlfeld R (1999) Activated human T cells, B cells and monocytes produced brain-derived neurotrophic factor in vitro and in inflammatory brain lesions: A neuroprotective role of inflammation? J Exp Med 189:865–870
Kerschensteiner M, Stadelmann C, Dechant G, Wekerle H, Hohlfeld R (2003) Neurotrophic cross-talk between the nervous and immune systems: Implications for neurological diseases. Ann Neurol 53:292–304
Kiefer R, Funa K, Schweitzer T, Jung S, Bourde O, Toyka KV, Hartung HP (1996) Transforming growth factor-β1 in experimental autoimmune neuritis: cellular localization and time course. Am J Pathol 148:211–223
Koski CL (1998) Immune interactions in the peripheral nervous system. In: Latov N, Wokke JHJ, Kelly JJ Jr (eds). Immunology and infectious diseases of the peripheral nerves. Cambridge University Press, Cambridge, pp 1–28
Kotter MR, Setzu A, Sim FJ, Van Rooijen N, Franklin RJM (2001) Macrophage depletion impairs oligodendrocyte remyelination following lysolecithin-induced demyelination. Glia 35:204–212
Krakowski M, Owens T (1996) Interferon-gamma confers resistance to experimental allergic encephalomyelitis. Eur J Immunol 26:1641–1646
Kuhlmann T, Bruck W (1999) Immunoglobulins induced myelin debris clearance by mouse macrophages. Neurosci Lett 19:191–194
Lambiase A, Bracci-Laudiero L, Bonini S, Bonini S, Starace G, D’Elios MM, De Carli M, Aloe L (1997) Human CD4+ T cell clones produce and release nerve growth factor and express high-affinity nerve growth factor receptors. J Allergy Clin Immunol 100:408–414
Latov N, Wokke JHJ, Kelly JJ Jr (1998) Immunology and infectious diseases of the peripheral nerves. Cambridge University Press, Cambridge
Lazarov-Spiegeler O, Salomon AS, Zeev-Brann AB, Hirschberg DL, Lavie V, Schwartz M (1996) Transplantation of activated macrophages overcomes central nervous system regrowth failure. FASEB J 10:1296–1302
Lazarov-Spiegeler O, Salomon AS, Schwartz M (1998) Peripheral nervestimulated macrophages simulate a peripheral nerve-like regenerative response in rat transected optic nerve. Glia 24:329–337
Lewin GR, Barde YA (1996) Physiology of the neurotrophins. Annu Rev Neurosci 19:289–317
Lilje O, Armati PJ (1997) The distribution and abundance of MHC and ICAM-1 on Schwann cells in vitro. J Neuroimmunol 77:75–84
Lindholm D, Heumann R, Meyer M, Thoenen H (1987) Interleukin-1 regulates synthesis of nerve growth factor in non-neuronal cells of rat sciatic nerve. Nature 330:658–659
Linker RA, Mäurer M, Gaupp S, Martini R, Holtmann B, Giess R, Rieckmann P, Lassman H, Toyka KV, Sendtner M, Gold R (2002) CNTF is a major protective factor in demyelinating CNS disease: A neurotrophic cytokine as modulator in neuroinflammation. Nature Med 8:620–624
Lisak RP, Bealmer B, Ragheb S (1994) Interleukin-1 alpha, but not interleukin 1-beta, is a co-mitogen for neonatal rat Schwann cells in vitro and acts via interleukin-1 receptors. J Neuroimmunol 55:171–177
Mancardi GL, Cadoni A, Zicca A, Schenone A, Tabaton A, De Martin A, Zaccheo D (1988) HLA-DR Schwann cell reactivity in peripheral neuropathies of different origins. Neurology 38:848–851
Marine JC, Topham DJ, McKay C, Wang D, Parganas E, Stravopodis E, Yoshimura A, Ihle JN (1999) SOCS1 deficiency causes a lymphocyte-dependent perinatal lethality. Cell 98:609–616
Martin R, McFarland HF, McFarlin DE (1992) Immunological aspects of demyelinating diseases. Annu Rev Immunol 10:153–187
Massa PT, ter Meulen V, Fontana A (1987) Hyperinducibility of Ia antigen on astrocytes correlates with strain-specific susceptibility to experimental autoimmune encephalomyelitis. Proc Natl Acad Sci USA 84:4219–4223
Masson JL, Jones JJ, Taniike M, Morelll P, Suzuki K, Matsushima GK (2000) Mature oligodendrocytes apoptosis precedes IGF-I production and oligodendrocyte progenitor accumulation and differentiation during demyelination/remyelination. J Neurosci Res 61:251–262
Masson JL, Suzuki K, Chaplin DD, Matsushima GK (2001) Interleukin-1β promotes repair of the CNS. J Neurosci 21:7046–7052
Matsumoto Y, Ohmori K, Fujiwara M (1992) Immune regulation by brain cells in the central nervous system by microglia but not astrocytes present myelin basic protein to encephalitogenic T cells under in vivo-mimicking conditions (1992). Immunology 76:209–216
Mears S, Schachner M, Brushart TM (2003) Antibodies to myelin-associated glycoprotein accelerate preferential motor reinnervation. J Peripher Nerv Syst 8:91–99
Melamed I, Kelleher CA, Franklin RA, Brodie C, Hempstead B, Kaplan D, Gelfand EW (1996) Nerve growth signal transduction in human B lymphocytes is mediated by gp 140trk. Eur J Immunol 26:1985–1992
Moalem G, Leibowitz-Amit R, Yoles E, Mor F, Cohen IR, Schwartz M (1999) Autoimmune T cells protect neurons from secondary degeneration after central nervous system axotomy. Nature Med 5:49–55
Mohan R, Edwards ET, Cupps TR, Oliverio PJ, Sandberg G, Crayton H, Richert JR, Siegel JN (2001) Demyelination occurring during anti-tumor necrosis factor alpha therapy for inflammatory arthritis. Arthritis Rheum 44:2862–2869
Mukhopadhyay G, Doherty P, Walsh FS, Crocker PR, Filbin MT (1994) A novel role for myelin-associated glycoprotein as an inhibitor of axonal regeneration. Neuron 13:757–767
Neumann H, Misgeld T, Matsumuro K, Wekerle H (1998) Neurotrophins inhibit major histocompatibility class II inducibility of microglia: involvement of the p75 neurotrophin receptor. Proc Natl Acad Sci 95:5779–5784
Otten U, Ehrhard P, Peck E (1989) Nerve growth factor induces growth and differentiation of human B lymphocytes. Proc Natl Acad Sci 86:10059–10063
Ousman SS, David S (2000) Lysophosphatidylcholine induces rapid recruitment and activation of macrophages in the adult mouse spinal cord. Glia 30:92–104
Oya T, Zhao YL, Takagawa K, Kawaguchi M, Shirakawa K,Yamauchi T, Sasahara M (2002) Platelet-derived growth factor-β expression induced after rat peripheral nerve injuries. Glia 38:303–312
Pachter JS, de Vries HE, Fabry Z (2003) The blood-brain barrier and its role in immune privilege in the Central Nervous System. J Neuropathol Exp Neurol 62:593–604
Panitch HS, Hirsch RL, Schindler J, Johnson KP (1987) Treatment of multiple sclerosis with gamma interferon: exacerbations associated with activation of the immune system. Neurology 37:1097–1102
Paz Soldan MM, Warrington AE, Bieber AJ, Ciric B, Van Keulen V, Pease LR, Rodríguez M (2003) Remyelination-promoting antibodies activate distinct Ca2+ influx pathways in astrocytes and oligodendrocytes: relationship to the mechanisms of myelin repair. Moll Cell Neurosci 22:14–24
Perry VH, Brown MC, Gordon S (1987) The macrophage response to central and peripheral injury: A possible role for macrophages in regeneration. J Exp Med 165:1218–1223
Pollard JD, Baverstocl J, McLeod JG (1987) Class II antigen expression and inflammatory cells in the Guillain-Barré syndrome. Ann Neurol 21:337–341
Poulsen FR, Lagord C, Courty J, Pedersen EB, Barritault D, Finsen B (2000) Increased synthesis of heparin affin regulatory peptide in the perforant path lesioned mouse hippocampal formation. Exp Brain Res 135:319–330
Prineas JW, McLeod JG (1976) Chronic relapsing polyneuritis. J Neurol Sci 27:427–458
Prineas JW (1981) Pathology of Guillain-Barre syndrome. Ann Neurol 9(Suppl):6–19
Rabchensky AG, Streitt WJ (1997) Grafting of cultured microglia cells into the lesioned spinal cord of adult rats enhances neurite outgrowth. J Neurosci Res 47:34–48
Ransohoff RR, Benveniste EN (1996) Cytokines and the CNS. CRC Press, Boca Raton
Ransohoff RM, Howe CL, Rodriguez M (2002) Growth factor treatment of demyelinating disease: at last, a leap into the light. Trends Immunol 23:512–516
Rapalino O, Lazarov-Spiegeler O, Agranov E, Velan GJ, Yoles E, Fraidakis M, Solomon A, Gepstein R, Katz A, Belkin M, Hadani M, Schwartz M (1998) Implantation of stimulated homologous macrophages results in partial recovery of paraplegic rats. Nature Med 4:814–821
Reichert F, Levitzky R, Rothshenker S (1996) Interleukin 6 in intact and injured mouse peripheral nerves. Eur J Neurosci 8:530–535
Robinson S, Miller RH (1999) Contact with central nervous system myelin inhibits oligodendrocyte progenitor maturation. Dev Biol 216:359–368
Rodriguez M, Lennon VA, Benveniste EN, Merril JE (1987) Remyelination by oligodendrocytes stimulated by antiserum to spinal cord. J Neuropathol Exp Neurol 46:84–95
Rotshenker S, Aamar S, Barak V (1992) Interleukin-1 activity in lesioned peripheral nerve. J Neuroimmunol 39:75–80
Saada A, Reichert F, Rotshenker S (1996) Granulocyte macrophage colony stimulating factor produced in lesioned peripheral nerves induces the up-regulation of cell surface expression of MAC-2 by macrophages and Schwann cells. J Cell Biol 133:159–167
Santambrogio L, Benedetti M, Caho MV, Muzaffar R, Kulig K, Gabell N, Hochwald G (1994) Nerve growth factor production by lymphocytes. J Immunol 153:4488–4495
Scherbel U, Raghupathi R, Nakamura M, Saatman KE, Trojanowski JQ, Neugebauer E,Marino MW, McIntosh TK (1999) Differential acute and chronic responses of tumor necrosis factor-deficient mice to experimental brain injury. Proc Natl Acad Sci 96:8721–8726
Schmidt B, Stoll G,Hartung HP, Heininger K, Schafer B, Toyka KV (1990) Macrophages but not Schwann cells express Ia antigen in experimental allergic neuritis. Ann Neurol 28:70–77
Schwartz M, Hauben E (2002) T-cell based therapeutic vaccination for spinal cord injury. Prog Brain Res 137:401–406
Sedgwick JD, Mossner R, Schwender S, ter Meulen V (1991) Major histocompatibility complex-expressing nonhematopoietic astroglial cells prime only CD8+ T lymphocytes: astroglial cells as perpetuators but not initiators of CD4+ T cell responses in the central nervous system. J Exp Med 173:1235–1246
Shamash S, Reichert F, Rotshenker S (2002) The Cytokine network of Wallerian degeneration: Tumor necrosis factor-α, and Interleukin-1β. J Neurosci 22:3052–3060
Shields SA, Gilson JM, Blakemore WF, Franklin RJM (1999) Remyelination occurs as extensively but more slowly in old rats compared to young rats following gliotoxin-induced CNS demyelination. Glia 28:77–83
Sicotte NL, Voskhul RR (2001) Onset of multiple sclerosis associated with anti-TNF therapy. Neurology 57:1885–1888
Sindern E, Schweppe K, Ossege LM, Mailin JP (1996) Potential role of transforming growth factor-β1 in terminating the immune response in patients with Guillain-Barré syndrome. J Neurol 243:264–268
Sobue G, Yamamoto M, Doyu M, Li M, Yasuda T, Mitsuma T (1998) Expression of mRNA for neurotrophins (NGF, BDNF, and NT-3) and their receptors (p75NGFR, trkB, and trkC) in human peripheral neuropathies. Neurochem Res 23:821–829
Stadelmann C, Kerschensteiner M, Misgeld T, Brück W, Hohlfeld R, Lassmann H (2002) BDNF and gp145trkB in multiple sclerosis brain lesions: neuroprotective interactions between immune and neuronal cells? Brain 125:75–85
Stoll G, Griffin JW, Li CY, Trapp BD (1989) Wallerian degeneration in the peripheral nervous system: Participation of both Schwann cells and macrophages in myelin degradation. J Neurocytol 18:671–683
Stoll G, Jung S, Van der Meide P, Hartung HP (1993) Tumor necrosis factor-alpha in immune mediated demyelination and Wallerian degeneration of the peripheral nervous system. J Neuroimmunol 45:175–182
The Lenercep Group (1999) TNF neutralization in MS: results of a randomized placebo-controlled multicenter study. Neurology 53:457–465
Thorpe LW, Perez-Polo JR (1987) The influence of nerve growth factor on in vitro proliferative response of rat spleen lymphocytes. J Neurosci Res 18:134–139
Torcia M, Bracci-Laudiero L, Lucibello M, Nencioni L, Labardi D, Rubartelli A, Cozzolino F, Aloe L, Garaci E (1996) Nerve growth factor is an autocrine survival factor for memory B lymphocytes. Cell 85:345–356
van Oosten BW, Barkhof F, Truyen L, Boringa JB, Bertelsmann FW, von Blomberg BM, Woody JN, Hartung HP, Polman CH (1996) Increased MRI activity and immune activation in two multiple sclerosis patients treated with the monoclonal anti-tumor necrosis factor antibody cA2. Neurology 47:1531–1534
Villoslada P,Hauser SL, Bartke I, Unger J, Heald N, Rosenberg D, Cheung SW, Mobley WC, Fisher S, Genain CP (2000) Human nerve growth factor protects common marmosets against autoimmune encephalomyelitis by switching the balance of T helper cell type 1 and 2 cytokines within the central nervous system. J Exp Med 191:1799–1806
Wagner R, Myers RR (1996) Schwann cells produce tumor necrosis factor alpha: expression in injured and non-injured nerves. Neuroscience 73:625–629
Warrington AE, Asakura K, Bieber AJ, Ciric B, Van Keulen V, Kaveri SV, Kyle RA, Pease LR, Rodriguez M (2000) Human monoclonal antibodies reactive to oligodendrocytes promote remyelination in a model of multiple sclerosis. Proc Natl Acad Sci 97:6820–6825
Warrington AE, Bieber AJ, van Keulen V, Ciric B, Pease LR, Rodriguez M (2004) Neuron-binding human monoclonal antibodies support central nervous system neurite extension. J Neuropathol Exp Neurol 63:461–473
Weber F, Meinl E, Aloisi F, Nevinny-Stickel C, Albert E, Wekerle H, Hohlfeld R (1994) Human astrocytes are only partially competent antigen presenting cells. Possible implications for lesion development in multiple sclerosis. Brain 117:59–69
Wei R, Jonakait GM (1999) Neurotrophins and the anti-inflammatory agents interleukin-4 (IL-4), IL-10, IL-11 and transforming growth factor beta-1 (TGF-beta 1) down-regulate T cell costimulatory molecules B7 and CD40 on cultured rat microglia. J Neuroimmunol 95:8–18
Weishaupt A, Gold R, Hartung H, Gaupp S, Brück W, Toyka KV (2000) Role of TNF-α in high-dose antigen therapy in experimental autoimmune neuritis: inhibition of TNF-α by neutralizing antibodies reduces T-cell apoptosis and prevents liver necrosis. J Neuropathol Exp Neurol 59:368–376
Willenborg DO, Fordham S, Bernard CC, Cowden WB, Ramshaw IA (1996) IFN-gamma plays a critical down-regulatory role in the induction and effector phase of myelin oligodendrocyte glycoprotein-induced autoimmune encephalomyelitis. J Immunol 157:3223–3227
Williams K, Ulvestad E, Antel JP (1994) B7/BB-1 antigen expression on adult microglia studied in vitro and in situ. Eur J Immunol 24:3031–3037
Windhangen A, Newcombe J, Dangond F, Strand C, Woodroofe MNB, Cuzner ML, Hafler DA (1995) Expression of costimulatory molecules B7-1 (CD80), B7-2 (CD86), and interleukin 12 cytokine in multiple sclerosis lesions. J Exp Med 182:1985–1996
Woodruff RH, Franklin RJM (1999) The expression of myelin protein mRNAs during remyelination of lysolecithin-induced demyelination. Neuropathol Appl Neurobiol 25:226–235
Yamamoto M, Sobue G, Li M, Arakawa Y, Mitsuma T, Kimata K (1993) Nerve growth factor (NGF), brain derived neurotrophic factor (BDNF) and low-affinity nerve growth factor receptor (LNGFR) mRNA levels in cultured rat Schwann cells; differential time-and-dose-dependent regulation by cAMP. Neurosci Lett 152:37–40
Yao DL, Liu X, Hudson LD, Webster HD (1995) Insulin-like growth factor I treatment reduces demyelination and up-regulates gene expression of myelin-related proteins in experimental autoimmune encephalomyelitis. Proc Natl Acad Sci USA 92:6190–6194
Yoles E, Hauben E, Palgi O, Agarnov E, Gothilf A, Cohen A, Kuchroo V, Cohen IR, Weiner HL, Schwartz M (2001) Protective autoimmunity is a physiological response to CNS trauma. J Neurosci 21:3740–3748
Zeev-Brann AB, Lazarov-Spiegler O, Brenner T, Schwartz M (1998) Differential effects of central and peripheral nerves on macrophages and microglia. Glia 23:181–190
Zettl UK, Mix E, Zielasek J, Stangel M, Hartung HP, Gold R (1997) Apoptosis of myelin-reactive T cells induced by reactive oxygen and nitrogen intermediates in vitro. Cell Immunol 178:1–8
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Correale, J., Villa, A. The neuroprotective role of inflammation in nervous system Injuries. J Neurol 251, 1304–1316 (2004). https://doi.org/10.1007/s00415-004-0649-z
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/s00415-004-0649-z