Abstract
Cr, Fe, Ce and W doped MoVTeNbO M2 phase catalysts were synthesized and used in the selective oxidation of propylene to acrylic acid (AA). Results show that the introduction of Cr, Fe, Ce and W substantially affects the physicochemical properties and catalytic performance of MoVTeNbO-based catalysts. Un-doped catalyst consists of M2 phase and TeO2, while Cr, Fe, Ce and W-doped catalysts are mainly composed of M2 and MoO3. It is indicated that doping of Cr, Fe, Ce and W can restrain the formation of TeO2, but favour the formation of MoO3. Un-doped, Cr and W-doped catalysts display irregular-shaped particles morphology, while Fe and Ce-doped catalysts display nanosheets morphology. In addition, the valence of superficial elements of catalysts changed greatly with the doping elements. For catalytic performance, in addition to Cr, the propylene conversion of the catalyst decreases obviously with doping of other elements, probably due to the drastically reduced specific surface area with doping of Fe, Ce and W. The existence of Cr and Ce can increase the selectivity to AA at all test temperatures (360–440 ℃), while Fe and W-doped catalysts only show higher selectivity than the un-doped one at high temperature of 420 and 440 ℃. It is illustrated that the catalysts with redox ability at relatively low temperature is more favorable for the selectivity to AA. Among them, Cr-doped catalyst shows the highest selectivity (85.3%) and yield (63.5%) of AA at test temperature of 380 ℃, which are 15.3 and 7.5% higher than that of un-doped catalyst, respectively.
Graphic Abstract
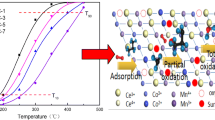
The M2 phase MoVTeNbO catalysts doped with Cr, Fe, Ce and W have been synthesized. It is demonstrated that the addition of Cr improves the stability of Te4+, and Cr-doped M2 phase shows excellent catalytic performance in the selective oxidation of propylene to acrylic acid.









Similar content being viewed by others
References
Korovchenko P et al (2008) M1 to M2 phase transformation and phase cooperation in bulk mixed metal Mo–V–M–O (M = Te, Nb) catalysts for selective ammoxidation of propane. Top Catal 50(1–4):43–51
Ishikawa S, Ueda W (2016) Microporous crystalline Mo–V mixed oxides for selective oxidations. Catal Sci Technol 6(3):617–629
Zhai Z et al (2016) Effects of catalyst crystal structure on the oxidation of propene to acrolein. Catal Today 261:146–153
Woo J et al (2016) A study of M1/M2 phase synergy in the MoVTe(Nb,Ta)O catalysts for propane ammoxidation to acrylonitrile. Appl Catal A Gen 515:179–189
Kong L et al (2016) Oxidative dehydrogenation of ethane to ethylene over Mo-incorporated mesoporous SBA-16 catalysts: the effect of MoO dispersion. Appl Catal A Gen 510:84–97
Ishchenko EV et al (2016) Effect of K and Bi doping on the M1 phase in MoVTeNbO catalysts for ethane oxidative conversion to ethylene. Appl Catal A Gen 514:1–13
Massó Ramírez A et al (2020) Optimizing reflux synthesis method of Mo-V-Te-Nb mixed oxide catalysts for light alkane selective oxidation. Catal Today 356:322–329
Ishchenko EV et al (2013) Role of MoVTeNb oxide catalyst constituent phases in propane oxidation to acrylic acid. Catal Sustain Energy 1:75–81
Ramli I et al (2011) Reflux method as a novel route for the synthesis of MoVTeNbOx catalysts for selective oxidation of propane to acrylic acid. J Mol Catal A Chem 342–343:50–57
Deniau B et al (2008) Effect of several cationic substitutions in the M1 active phase of the MoVTeNbO catalysts used for the oxidation of propane to acrylic acid. J Catal 260(1):30–36
Hävecker M et al (2012) Surface chemistry of phase-pure M1 MoVTeNb oxide during operation in selective oxidation of propane to acrylic acid. J Catal 285(1):48–60
Oh K, Woo S (2008) Effect of preparation and reaction condition on the catalytic performance of Mo–V–Te–Nb catalysts for selective oxidation of propane to acrylic acid by high-throughput methodology. Catal Today 137(1):61–70
Tu X et al (2018) Controlled silylation of MoVTeNb mixed oxide catalyst for the selective oxidation of propane to acrylic acid. Appl Catal A Gen 549:152–160
Song Y-Y, Wang G-C (2018) Theoretical study of propylene epoxidation over Cu2O(111) surface: activity of O2–, O–, and O2– species. J Phys Chem C 122(37):21500–21513
Lei Y et al (2010) Increased silver activity for direct propylene epoxidation via subnanometer size effects. Science 328(5975):224–228
Wang G et al (2006) A systematic theoretical study of water dissociation on clean and oxygen-preadsorbed transition metals. J Catal 244(1):10–16
Tompos A et al (2021) Combinatorial optimization and synthesis of multiple promoted MoVNbTe catalysts for oxidation of propane to acrylic acid. Catal Today 363:45–54
Quintana-Solórzano R et al (2020) On the simultaneous effect of temperature, pressure, water content and space–time on acrylic acid production from propane. Fuel 282:118852–118857
Li S et al (2020) Facile sub-/supercritical water synthesis of nanoflake MoVTeNbOx-mixed metal oxides without post-heat treatment and their catalytic performance. RSC Adv 10(65):39922–39930
Roosta H et al (2018) Effects of chemical modification of PVA by acrylamide, methacrylamide and acrylonitrile on the growth rate of gas hydrate in methane–propane–water system. J Mol Liq 253:259–269
Trifirò F (2021) Some history on the new ways of synthesis of nitriles. Catal Today 363:10–14
Planer-Friedrich G (2004) Structural aspects of the M1 and M2 phases in MoVNbTeO propane ammoxidation catalysts. Intl J Struct Phys Chem Aspects Crystall Mater 219(3):152–165
Kum SS et al (2011) Improved performance of Mo-V-Te-Nb-Ox catalysts prepared from a solution containing drying control chemical additives in propane oxidation to acrylic acid. Korean Chem Eng 28(6):1364–1371
Desanto P et al (2003) Structural characterization of the orthorhombic phase M1 in MoVNbTeO propane ammoxidation catalyst. Top Catal 23(1):23–38
Gaffney AM et al (2020) Toward concurrent engineering of the M1-based catalytic systems for oxidative dehydrogenation (ODH) of alkanes. Top Catal 63(19–20):1667–1681
Melzer D et al (2020) On the promoting effects of Te and Nb in the activity and selectivity of M1 MoV-oxides for ethane oxidative dehydrogenation. Top Catal 63(19–20):1754–1764
Häggblad R et al (2008) Substituted Mo–V(Ti)–Te(Ce)-oxide M2 catalysts for propene ammoxidation. Top Catal 50(1–4):52–65
Holmberg J et al (2007) Catalytic and structural effects of W-substitution in M2 Mo-V-Te-oxide for propene ammoxidation. Catal Today 128(3–4):153–160
Hernández-Morejudo S et al (2015) Preparation, characterization and catalytic behavior for propane partial oxidation of Ga-promoted MoVTeO catalysts. Appl Catal A Gen 504:51–61
Kardash TY et al (2018) The evolution of the M1 local structure during preparation of VMoNbTeO catalysts for ethane oxidative dehydrogenation to ethylene. RSC Adv 8(63):35903–35916
Lazareva EV et al (2021) Oxidative dehydrogenation of ethane over M1 MoVNbTeO catalysts modified by the addition of Nd, Mn, Ga or Ge. Catal Today 361:50–56
Biswas P et al (2010) Ruthenium and gold-doped M1 phase MoVNbTeO catalysts for propane ammoxidation to acrylonitrile. Catal Commun 12(1):58–63
Hibbitts D, Neurock M (2016) Promotional effects of chemisorbed oxygen and hydroxide in the activation of C–H and O–H bonds over transition metal surfaces. Surf Sci 650:210–220
Wang J, Wang G-C (2018) Promotion effect of methane activation on Cu(111) by the surface-active oxygen species: a combination of DFT and ReaxFF study. J Phys Chem C 122(30):17338–17346
Ivars-Barceló F et al (2014) Understanding effects of activation-treatments in K-free and K-MoVSbO bronze catalysts for propane partial oxidation. Catal Today 238:41–48
Andrushkevich TV et al (2015) Propane ammoxidation on Bi promoted MoVTeNbOx oxide catalysts: effect of reaction mixture composition. Appl Catal A Gen 506:109–117
Wang G et al (2015) Promotional effect of cerium on Mo–V–Te–Nb mixed oxide catalyst for ammoxidation of propane to acrylonitrile. Fuel Process Technol 130:71–77
Grasselli RK et al (2010) Enhancement of acrylic acid yields in propane and propylene oxidation by selective P doping of MoV(Nb)TeO-based M1 and M2 catalysts. Catal Today 157(1–4):33–38
Xu A et al (2013) An outstanding Cr-doped catalyst for selective oxidation of propane to acrylic acid. Chin J Catal 34(12):2183–2191
Yun YS et al (2014) Rational design of a bifunctional catalyst for the oxydehydration of glycerol: a combined theoretical and experimental study. ACS Catal 5(1):82–94
Aixin et al (2013) An outstanding Cr-doped catalyst for selective oxidation of propane to acrylic acid. Chin J Catal 34(12):2183-2191
Chieregato A et al (2012) Glycerol oxidehydration into acrolein and acrylic acid over W–V–Nb–O bronzes with hexagonal structure. Catal Today 197(1):58–65
Florea M et al (2006) High surface area Mo–V–Te–Nb–O catalysts: preparation, characterization and catalytic behaviour in ammoxidation of propane. Catal Today 112(1–4):139–142
Grasselli RK et al (2003) Multifunctionality of active centers in (amm)oxidation catalysts: from Bi–Mo–Ox to Mo–V–Nb–(Te, Sb)–Ox. Top Catal 23(1):5–22
Wang G et al (2009) Synthesis of molybdenum oxide nanoplatelets during crystallization of the precursor gel from its hybrid nanocomposites. Chem Mater 19(5):979–981
Valente JS et al (2014) Chemical, structural, and morphological changes of a MoVTeNb catalyst during oxidative dehydrogenation of ethane. ACS Catal 4(5):1292–1301
Botto IL et al (1997) (NH4)6[TeMo6O24]·7H2O Anderson phase as precursor of the TeMo5O16 catalytic phase: thermal and spectroscopic studies. Mater Chem Phys 47(1):37–45
Brito JL et al (1989) Temperature-programmed reduction of Ni-Mo oxides. J Mater Sci 24(2):425–431
Chu B et al (2015) Performance of phase-pure M1 MoVNbTeOx catalysts by hydrothermal synthesis with different post-treatments for the oxidative dehydrogenation of ethane. Appl Catal A Gen 498:99–106
Choi JG, Thompson LT (1996) XPS study of as-prepared and reduced molybdenum oxides. Appl Surf Sci 93(2):143–149
Ozkar S et al (1992) Photooxidation of hexacarbonylmolybdenum(0) in sodium zeolite Y to yield redox-interconvertible molybdenum(VI) oxide and molybdenum(IV) oxide monomers. Chem Mater 4(6):1380–1388
Garbassi F et al (1981) XPS study of tellurium-niobium and tellurium-tantalum oxide systems. J Electron Spectros Relat Phenomena 22(2):95–107
Ozer N et al (1995) Characterization of sol-gel deposited niobium pentoxide films for electrochromic devices. Sol Energy Mater Sol Cells 36(4):433–443
Grasselli RK (2014) Site isolation and phase cooperation: two important concepts in selective oxidation catalysis: a retrospective. Catal Today 238:10–27
Grasselli RK (2005) Selectivity issues in (amm)oxidation catalysis. Catal Today 99(1–2):23–31
Grasselli RK et al (2006) Active centers, catalytic behavior, symbiosis and redox properties of MoV(Nb,Ta)TeO ammoxidation catalysts. Top Catal 38(1–3):7–16
Acknowledgements
The authors gratefully acknowledge the financial supports by the National Natural Science Foundation of China (No. 21706165), Scientific Research Fund of Liaoning Provincial Education Department, China (No. LQ2019007), LiaoNing Revitalization Talents Program (No. XLYC2002001), and Natural Science Foundation of Liaoning Province, China (No. 2021-MS-255).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
On behalf of all authors, the corresponding author states that there is no conflict of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Li, S., Lu, Z., Yan, Y. et al. The Structure and Catalytic Properties of MoVTeNbO Catalysts Modified by Adding Cr, Fe, Ce and W. Catal Surv Asia 26, 58–67 (2022). https://doi.org/10.1007/s10563-021-09346-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10563-021-09346-4