Abstract
Purpose
18F-fluorodeoxyglucose (FDG) uptake on positron emission tomography (PET) is related to the biological parameters and prognosis of breast cancer. However, whether whole-body PET (WBPET) and dedicated breast PET (DbPET) can reflect the tumor microenvironment is unclear. This study investigated the relationship between stromal tumor-infiltrating lymphocytes (TILs) and maximum standardized uptake value (SUVmax) in WBPET and DbPET.
Methods
A total of 125 invasive breast cancers underwent WBPET and ring-type DbPET and resected specimens were pathologically assessed. The impact of SUVmax on the tumor biological parameters and TILs was retrospectively evaluated. SUVmax was classified as high and low relative to the median values (WBPET-SUVmax: 2.2 and DbPET-SUVmax: 6.0).
Results
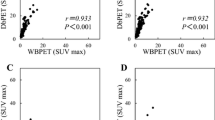
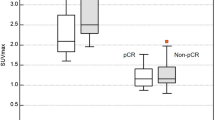
SUVmax correlated with tumor size, nuclear grade, Ki-67 labeling index, and TILs in both WBPET and DbPET (all p < 0.001). In multiple linear regression analysis, tumor size, Ki-67 labeling index, and TILs predicted SUVmax in WBPET and DbPET. The cutoff values of tumor size, Ki-67 labeling index, and TILs predicting high SUVmax were 20 mm, 20%, and 20%, respectively. In multivariate analysis, the predictive factors for high SUVmax were tumor size and Ki-67 labeling index for WBPET and tumor size and TILs for DbPET. High SUVmax in DbPET was related to high numbers of TILs after propensity score matching analysis; however, WBPET was not (p = 0.007 and p = 0.624, respectively).
Conclusions
Both SUVmax values in WBPET and DbPET predicted TIL concentration of the primary breast cancer. In DbPET, SUVmax represented the immune microenvironment after adjusting for tumor biological factors.



Similar content being viewed by others
Abbreviations
- CTLA-4:
-
Cytotoxic T-lymphocyte-associated protein 4
- DbPET:
-
Dedicated breast positron emission tomography
- ER:
-
Estrogen receptor
- FDG:
-
18F-fluorodeoxyglucose
- FOV:
-
Field of view
- FOXP3:
-
Forkhead box P3
- HER2:
-
Human epidermal growth factor receptor 2
- IC-NST:
-
Invasive carcinoma of no special type
- OR:
-
Odds ratio
- PD-1:
-
Programmed cell death-1
- PET:
-
Positron emission tomography
- SUVmax:
-
Maximum standardized uptake value
- TIL:
-
Tumor-infiltrating lymphocytes
- TNBC:
-
Triple-negative breast cancer
- WBPET:
-
Whole-body positron emission tomography
References
Nishikawa H, Sakaguchi S (2014) Regulatory T cells in cancer immunotherapy. Curr Opin Immunol 27:1–7. https://doi.org/10.1016/j.coi.2013.12.005
Stanton SE, Adams S, Disis ML (2016) Variation in the incidence and magnitude of tumor-infiltrating lymphocytes in breast cancer subtypes: a systematic review. JAMA Oncol 2(10):1354–1360. https://doi.org/10.1001/jamaoncol.2016.1061
Denkert C, von Minckwitz G, Darb-Esfahani S, Lederer B, Heppner BI, Weber KE, Budczies J et al (2018) Tumour-infiltrating lymphocytes and prognosis in different subtypes of breast cancer: a pooled analysis of 3771 patients treated with neoadjuvant therapy. Lancet Oncol 19(1):40–50. https://doi.org/10.1016/s1470-2045(17)30904-x
Dieci MV, Mathieu MC, Guarneri V, Conte P, Delaloge S, Andre F, Goubar A (2015) Prognostic and predictive value of tumor-infiltrating lymphocytes in two phase III randomized adjuvant breast cancer trials. Ann Oncol 26(8):1698–1704. https://doi.org/10.1093/annonc/mdv239
Palsson-McDermott EM, O’Neill LA (2013) The Warburg effect then and now: from cancer to inflammatory diseases. BioEssays 35(11):965–973. https://doi.org/10.1002/bies.201300084
Lee S, Choi S, Kim SY, Yun MJ, Kim HI (2017) Potential utility of FDG PET-CT as a non-invasive tool for monitoring local immune responses. J Gastric Cancer 17(4):384–393. https://doi.org/10.5230/jgc.2017.17.e43
Lopci E, Toschi L, Grizzi F, Rahal D, Olivari L, Castino GF, Marchetti S et al (2016) Correlation of metabolic information on FDG-PET with tissue expression of immune markers in patients with non-small cell lung cancer (NSCLC) who are candidates for upfront surgery. Eur J Nucl Med Mol Imaging 43(11):1954–1961. https://doi.org/10.1007/s00259-016-3425-2
Masumoto N, Kadoya T, Sasada S, Emi A, Arihiro K, Okada M (2018) Intratumoral heterogeneity on dedicated breast positron emission tomography predicts malignancy grade of breast cancer. Breast Cancer Res Treat. https://doi.org/10.1007/s10549-018-4791-1
MacDonald L, Edwards J, Lewellen T, Haseley D, Rogers J, Kinahan P (2009) Clinical imaging characteristics of the positron emission mammography camera: PEM Flex Solo II. J Nucl Med 50(10):1666–1675. https://doi.org/10.2967/jnumed.109.064345
Hammond ME, Hayes DF, Dowsett M, Allred DC, Hagerty KL, Badve S, Fitzgibbons PL et al (2010) American Society of Clinical Oncology/College Of American Pathologists guideline recommendations for immunohistochemical testing of estrogen and progesterone receptors in breast cancer. J Clin Oncol 28(16):2784–2795. https://doi.org/10.1200/jco.2009.25.6529
Wolff AC, Hammond ME, Hicks DG, Dowsett M, McShane LM, Allison KH, Allred DC et al (2013) Recommendations for human epidermal growth factor receptor 2 testing in breast cancer: American Society of Clinical Oncology/College of American Pathologists clinical practice guideline update. J Clin Oncol 31(31):3997–4013. https://doi.org/10.1200/jco.2013.50.9984
Salgado R, Denkert C, Demaria S, Sirtaine N, Klauschen F, Pruneri G, Wienert S et al (2015) The evaluation of tumor-infiltrating lymphocytes (TILs) in breast cancer: recommendations by an International TILs Working Group 2014. Ann Oncol 26(2):259–271. https://doi.org/10.1093/annonc/mdu450
Kanda Y (2013) Investigation of the freely available easy-to-use software ‘EZR’ for medical statistics. Bone Marrow Transplant 48(3):452–458. https://doi.org/10.1038/bmt.2012.244
Ohara M, Shigematsu H, Tsutani Y, Emi A, Masumoto N, Ozaki S, Kadoya T et al (2013) Role of FDG-PET/CT in evaluating surgical outcomes of operable breast cancer–usefulness for malignant grade of triple-negative breast cancer. Breast (Edinburgh, Scotland) 22(5):958–963. https://doi.org/10.1016/j.breast.2013.05.003
Kadoya T, Aogi K, Kiyoto S, Masumoto N, Sugawara Y, Okada M (2013) Role of maximum standardized uptake value in fluorodeoxyglucose positron emission tomography/computed tomography predicts malignancy grade and prognosis of operable breast cancer: a multi-institute study. Breast Cancer Res Treat 141(2):269–275. https://doi.org/10.1007/s10549-013-2687-7
Aogi K, Kadoya T, Sugawara Y, Kiyoto S, Shigematsu H, Masumoto N, Okada M (2015) Utility of (18)F FDG-PET/CT for predicting prognosis of luminal-type breast cancer. Breast Cancer Res Treat 150(1):209–217. https://doi.org/10.1007/s10549-015-3303-9
Akimoto E, Kadoya T, Kajitani K, Emi A, Shigematsu H, Ohara M, Masumoto N et al (2018) Role of (18)F-PET/CT in predicting prognosis of patients with breast cancer after neoadjuvant chemotherapy. Clin Breast Cancer 18(1):45–52. https://doi.org/10.1016/j.clbc.2017.09.006
Rousseau C, Devillers A, Sagan C, Ferrer L, Bridji B, Campion L, Ricaud M et al (2006) Monitoring of early response to neoadjuvant chemotherapy in stage II and III breast cancer by [18F]fluorodeoxyglucose positron emission tomography. J Clin Oncol 24(34):5366–5372. https://doi.org/10.1200/jco.2006.05.7406
Sasada S, Masumoto N, Goda N, Kajitani K, Emi A, Kadoya T, Okada M (2018) Dedicated breast PET for detecting residual disease after neoadjuvant chemotherapy in operable breast cancer: a prospective cohort study. Eur J Surg Oncol 44(4):444–448. https://doi.org/10.1016/j.ejso.2018.01.014
Fowler AM (2014) A molecular approach to breast imaging. J Nucl Med 55(2):177–180. https://doi.org/10.2967/jnumed.113.126102
Garcia Hernandez T, Vicedo Gonzalez A, Ferrer Rebolleda J, Sanchez Jurado R, Rosello Ferrando J, Brualla Gonzalez L, Granero Cabanero D et al (2016) Performance evaluation of a high resolution dedicated breast PET scanner. Med Phys 43(5):2261. https://doi.org/10.1118/1.4945271
Nishimatsu K, Nakamoto Y, Miyake KK, Ishimori T, Kanao S, Toi M, Togashi K (2017) Higher breast cancer conspicuity on dbPET compared to WB-PET/CT. Eur J Radiol 90:138–145. https://doi.org/10.1016/j.ejrad.2017.02.046
Iima M, Nakamoto Y, Kanao S, Sugie T, Ueno T, Kawada M, Mikami Y et al (2012) Clinical performance of 2 dedicated PET scanners for breast imaging: initial evaluation. J Nucl Med 53(10):1534–1542. https://doi.org/10.2967/jnumed.111.100958
Acknowledgements
We thank Kazushi Marukawa and Masatsugu Tsujimura of Chuden Hospital for providing PET examination data.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Sasada, S., Shiroma, N., Goda, N. et al. The relationship between ring-type dedicated breast PET and immune microenvironment in early breast cancer. Breast Cancer Res Treat 177, 651–657 (2019). https://doi.org/10.1007/s10549-019-05339-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10549-019-05339-0