Abstract
Purpose
The objective of this study is to understand an impact of financial burden on the adjuvant hormonal therapy (AHT) adherence and persistence for insured women aged 18–64 with early breast cancer in Texas.
Methods
We conducted a retrospective cohort study using claims data for population insured by Blue Cross Blue Shield of Texas from the year 2008 to 2013. Outcomes include adherence to adjuvant hormonal therapy, which was measured by medication possession ratio and persistence on AHT, which is the duration of time from initiation to discontinuation of therapy. Multivariate logistic regression models with repeated regional-level adjustments were used to explore the odds of AHT adherence. Cox proportional hazards model was conducted to assess time to the first 90+-day gap for persistence and a Kaplan–Meier curve were used to estimate probabilities to calculate the percentages of women who experienced 90+-day gaps in AHT.
Results
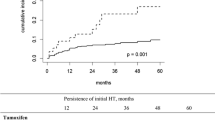
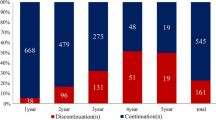
Of the 938 women in the cohort, 627 (66.8%) initiated the treatment. By year 1, 66.9% of women were adherent to the therapy, and by year 5, only 29% of those were adherent. The percentage of women with no gap in therapy greater than 90 days was 80.8%. Both higher out-of-pocket costs spent on all prescription drugs except AHT and AHT-specific out-of-pocket costs were negatively associated with adherence to AHT as well as continuing AHT as recommended.
Conclusions
Financial burdens including both non-AHT medication and AHT-specific out-of-pocket costs were significantly associated with adherence and persistence to the therapy.



Similar content being viewed by others
References
Yung RL, Hassett MJ, Chen K, Gesten FC, Roohan PJ, Boscoe FP et al (2012) Initiation of adjuvant hormone therapy by medicaid insured women with nonmetastatic breast cancer. J Natl Cancer Inst 14:1102–1111
Owusu C, Buist DSM, Field TS, Lash TL, Thwin SS, Geiger AM et al (2008) Predictors of tamoxifen discontinuation among older women with estrogen receptor—breast cancer. J Clin Oncol 26:549–555
Hershman DL, Kushi LH, Shao T, Buono D, Kershenbaum A, Tsai WY et al (2010) Early discontinuation and nonadherence to adjuvant hormonal therapy in a cohort of 8769 early-stage breast cancer patients. J Clin Oncol 28:4120–4128
Vrijens France, Stordeur Sabine, Beirens Koen, Devriese Stephan, Van Eycken Elizabeth, Vlayen Joan (2012) Effect of hospital volume on processes of care and 5-year survival after breast cancer: a population-based study on 25000 women. The Breast 21(3):261–266
Measring the Quality of Cancer Care. The National Initiative for Cancer Care Quality (NICCQ). 2006. RAND. www.rand.org as a public service of the RAND Corporation
Aday LA, Begley C, Lairson D, Balkrishnan R (2004) Evaluating the healthcare system: effectiveness, efficiency and equity, 3rd edn. Health Administration Press, Chicago
Partridge AH (2003) Nonadherence to adjuvant tamoxifen therapy in women with primary breast cancer. J Clin Oncol 21(4):602–606. https://doi.org/10.1200/jco.2003.07.071
Nekhlyudov L, Li L, Ross-Degnan D, Wagner AK (2011) Five-year patterns of adjuvant therapy use, persistence, and adherence among insured women with early-stage breast cancer. Breast Cancer Res Treat 130(2):681
Kimmick G, Anderson R, Camacho F, Bhosle M, Hwang W, Balkrishnan R (2009) Adjuvant hormonaltherapy use among insured, low-income women with breast cancer. J Clin Oncol 27(21):3445–3451
Cohen R, Gindi R, Kirzinger W. Financial burden of medical care: early release of estimates from the national health interview survey. http://www.cdc.gov/nchs/data/nhis/earlyrelease/financial_burden_of_medical_care_032012.pdf. Accessed 15 Jan 2014
Goldman DP, Joyce GF, Lawless G, Crown WH, Willey V (2006) Benefit design and specialty drug use. Health Affairs 25(5):1319–1331
Farias AJ, Du XL (2016) The association between out-of-pocket costs, race/ethnicity, and adjuvant endocrine therapy adherence among Medicare patients with breast cancer. J Clin Oncol 35(1):86–95
Biggers et al (2016) Medicare D subsidies and racial disparities in persistence and adherence with hormonal therapy. J Clin Oncol 34(36):4398–4404
Nattinger AB, Laud PW, Bajorunaite R, Sparapani RA, Freeman JL (2004) An algorithm for the use of Medicare claims data to identify women with incident breast cancer. Health Serv Res 39(6Pt 1):1733–1749
Cramer JA, Roy A, Burrell A, Fairchild CJ et al (2007) Medication compliance and persistence: terminology and definitions. Int Soc Pharma Outcomes Res (ISPOR) 11(1):44–47
Neugut AI, HIllyer GC, Kushi LH, LAmerato L, Leoce N, Nathanson DS et al (2012) Non-initiation of adjuvant hormonal therapy in women with hormone receptor positive breast cancer: the breast cancer quality of care study (B-QUAL). Breast Cancer Res Treat 134(1):419–428
Lash TL, Fox MP, Westrup JL, Fink AK, Silliman RA (2006) Adherence to tamoxifen over the five-year course. Breast Cancer Res Treat 99:215–220
Sedjo RL, Devine S (2011) Predictors of non-adherence to aromatase inhibitors among commercially insured women with breast cancer. Breast Cancer Research Treat Vol 125:191–200
Kimmick G, Anderson R, Camacho F, Bhosle M, Hwang W, Balkrishnan R (2009) Adjuvant hormonal therapy use among insured, low-income women with breast cancer. J Clin Oncol 27:3445–3451
Barron TI, Connolly R, Bennett K, Freely J, Kennedy J (2007) Early discontinuation of tamoxifen: a lesson for oncologists. Cancer 109:832–839
Cluse C, Rey D, Huiart L, BenDiane MK, Bouhnik AD, Berenger C et al (2012) Adjuvant endocrine therapy with tamoxifen in young women with breast cancer: determinants of interruptions vary over time. Ann Oncol 23:882–890
Charlson ME, Pompei P, Ales KL, MacKenzie CR (1987) A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis 40(5):373–383
The dartmouth atlas of health care. http://www.dartmouthatlas.org/data/region/
Neugut AI, Subar M, Wilde ET et al (2011) Association between prescription co-payment amount and compliance with adjuvant hormonal therapy in women with early-stage breast cancer. J Clin Oncol 29(18):2534–2542
Hershman DL, Tsui J, Meyer J et al (2014) The change from brand-name to generic aromatase inhibitors and hormone therapy adherence for early-stage breast cancer. J Natl Cancer Inst 106(11):dju319
Karnon J (2006) Aromatase inhibitors in breast cancer: a review of cost considerations and cost effectiveness. Pharmacoeconomics 24(3):215–232
Doughty JC (2011) When to start an aromatase inhibitor: now or later? J Surg Oncol 103(7):730–738
Arimidex, Tamoxifen, Alone or in Combination Trialists’ Group, Buzdar A, Howell A, Cuzick J, Wale C, Distler W, et al (2006) Comprehensive side-effect profile of anastrozole and tamoxifen as adjuvant treatment for early-stage breast cancer: Long-term safety analysis of the ATAC trial. Lancet Oncol 7(8):633–43
Duffy S, Jackson TL, Lansdown M, Philips K, Wells M, Clack G et al (2010) The ATAC adjuvant breast-cancer trial: six-year results of the endometrial subprotocol. J Obstet Gynaecol 30(6):596–604
Goldman DP, Joyce GF, Escarce JJ et al (2004) Pharmacy benefits and the use of drugs by the chronically ill. JAMA 291:2344–2350
Hadji P (2010) Improving compliance and persistence to adjuvant tamoxifen and aromatase inhibitor therapy. Crit Rev Oncol Hematol 73:156–166
Hede K (2009) Increase in oral cancer drugs raises thorny issues for oncology practices. J Natl Cancer Inst 101(22):1534–1536
Vanchieri C (2005) When will the U.S. flinch at cancer drug prices? J Natl Cancer Inst 97(9):624–626
Benson R, Wilson C, Williams MV (1998) Comments on Costs of treating advanced colorectal cancer, Ross et al., Eur J Cancer. 1996;32A:S13–S17. Eur J Cancer 34:593–594
Ruddy K, Mayer E, Partridge A (2009) Patient adherence and persistence with oral anticancer treatment. CA Cancer J Clin 59(1):56–66
Hershman DL, Shao T, Kushi LH et al (2011) Early discontinuation and non-adherence to adjuvant hormonal therapy are associated with increased mortality in women with breast cancer. Breast Cancer Res Treat 126:529. https://doi.org/10.1007/s10549-010-1132-4
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
None.
Rights and permissions
About this article
Cite this article
Kim, J., Rajan, S.S., Du, X.L. et al. Association between financial burden and adjuvant hormonal therapy adherence and persistent use for privately insured women aged 18–64 years in BCBS of Texas. Breast Cancer Res Treat 169, 573–586 (2018). https://doi.org/10.1007/s10549-018-4704-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10549-018-4704-3