Abstract
Mutations in the SEPSECS gene are associated with pontocerebellar hypoplasia type 2D. Pontocerebellar hypoplasia (PCH) is a heterogeneous group of rare autosomal recessive neurodegenerative disorders, mainly affecting pons and cerebellum. Patients have severe motor and cognitive impairments and often die during infancy. Here, we report a 23-year-old woman with slowly progressive cerebellar ataxia and cognitive impairment, in whom a homozygous missense mutation in the SEPSECS gene (c.1321G>A; p.Gly441Arg) was identified with whole exome sequencing. Our findings underline that defects in selenoprotein synthesis can also result in milder cerebellar atrophy presenting at a later age.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
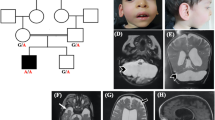
A 23-year-old woman presented with a history of intellectual disability, ataxia and progressive decline of motor function after initial normal development. Neurological examination revealed microcephaly (head circumference is 50.5 cm; −3.6SD), horizontal nystagmus, evident dysmetria and a broad based gait with postural instability. Magnetic resonance imaging (MRI) of the brain at the age of 16 years showed mild cerebellar atrophy (not shown), which had progressed at the age of 23 (Fig. 1b and Fig. 1a shows control image for comparison). Several metabolic (e.g. congenital disorders of glycosylation type 1a) and genetic (e.g. Friedreich ataxia) disorders were excluded (see Supplementary material for extended case description).
Brain MRI showing cerebellar atrophy in our patient, compared to a control and a PCH2A patient. (a) T1-weighted midsagittal image of a control. (b) T1-weighted midsagittal image of our patient, aged 23. Enlarged extracerebellar liquor spaces indicate atrophy of cerebellar vermis. The pons is normal. (c) Midsaggital MPR-image of a PCH2A patient at 6 months of age shows severe flattening of the ventral pons and hypoplasia of the cerebellar vermis. (d) MPR coronal image of control. (e) MPR coronal image or our patient shows atrophy of cerebellar hemispheres. (f) MPR coronal image of PCH2A patient indicates severe cerebellar hypoplasia with relative sparing of the vermis, resulting in a typical dragonfly pattern
Whole exome sequencing (trio analysis) revealed a homozygous missense variant: c. 1321G > A; p.(Gly441Arg) in exon 11 of the O-Phosphoseryl-tRNA selenocysteine tRNA synthase (SEPSECS, NM_016955.3) gene in the patient. Both parents were heterozygous carriers of this variant, that was predicted pathogenic by various in silico prediction programs (see Supplementary material).
SEPSECS mutations are associated with pontocerebellar hypoplasia type 2D (PCH2D). PCH2 is a prenatal onset neurodegenerative disorder characterized by severe hypoplasia of cerebellum and pons (Fig. 1c). PCH2D is a very rare subtype of PCH2 characterized by profound intellectual disability, progressive microcephaly, spasticity, epilepsy and progressive cerebral and cerebellar atrophy (Agamy & Zeev 2010). Additional features like axonal neuropathy, optic nerve atrophy, secondary mitochondrial dysfunction and early onset epileptic encephalopathy with burst suppression were later reported in patients with SEPSECS mutations (Anttonen et al 2015; Pavlidou et al 2016; Olson et al 2017). Disease onset was early and psychomotor development was severely delayed or absent in these patients. Our findings indicate that SEPSECS mutations can also give rise to milder and later onset neurodegeneration. It is currently unknown why our patient’s phenotype is so much milder. Possibly, the Gly441Arg missense mutation, which is located in the last exon of SEPSECS, results in a SEPSECS protein with a higher residual activity. This milder clinical presentation of SEPSECS mutations should be kept in mind in clinical care and when interpreting SEPSECS variants identified by whole exome sequencing.
References
Agamy O, Ben ZB, Lev D et al (2010) Mutations disrupting Selenocysteine formation cause progressive Cerebello-cerebral atrophy. Am J Hum Genet 87(4):538–544
Anttonen AK, Hilander T, Linnankivi T et al (2015) Selenoprotein biosynthesis defect causes progressive encephalopathy with elevated lactate. Neurology 85(4):306–315
Olson HE, Kelly M, LaCoursiere CM et al (2017) Genetics and genotype–phenotype correlations in early onset epileptic encephalopathy with burst suppression. Ann Neurol 81(3):419–429
Pavlidou E, Salpietro V, Phadke R, et al (2016) Pontocerebellar hypoplasia type 2D and optic nerve atrophy further expand the spectrum associated with selenoprotein biosynthesis deficiency. Eur J Paediatr Neurol 20(3):483–488
Acknowledgements
We would like to thank the patient and her family for participation in this study. This work was supported by the Joshua Deeth foundation.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
None.
Informed consent
All procedures followed were in accordance with the ethical standards of the responsible committee on human experimentation (institutional and national) and with the Helsinki declaration of 1975, as revised in 2000. Informed consent was obtained from the patients’ parents for being included in the study.
Additional information
Responsible Editor: Ina Knerr
Electronic supplementary material
ESM 1
(DOC 26 kb)
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
van Dijk, T., Vermeij, JD., van Koningsbruggen, S. et al. A SEPSECS mutation in a 23-year-old woman with microcephaly and progressive cerebellar ataxia. J Inherit Metab Dis 41, 897–898 (2018). https://doi.org/10.1007/s10545-018-0151-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10545-018-0151-x