Abstract
Background
Variants in the CASK gene result in a wide range of observed phenotypes in humans, such as FG Syndrome 4 and intellectual disabilities. Intellectual developmental disorder with microcephaly and pontine and cerebellar hypoplasia (MICPCH) is an X-linked disorder that affects females and is characterized by severely impaired intellectual development and variable degrees of pontocerebellar hypoplasia. Variants in CASK are the main genetic cause of MICPCH. Variants in CASK can explain most patients with MICPCH, but there are still some patients whose disease aetiology cannot be explained.
Case presentation
An 11-month-old female diagnosed with MICPCH exhibited general developmental delays, microcephaly, and cerebellar hypoplasia. Whole-exome sequencing (WES) was used to find a novel heterozygous missense variant (NM_003688.3: c.638T>G) of CASK in this patient. Strikingly, this variant reduced the expression of CASK at the protein level but not at the mRNA level. By using protein structure prediction analysis, this study found that the amino acid change caused by the variant resulted in further changes in the stability of the protein structure, and these changes caused the downregulation of protein expression and loss of protein function.
Conclusion
In this study, we first reported a novel heterozygous pathogenic variant and a causative mechanism of MICPCH. The amino acid change cause by this variant led to changes in the protein structure and a decrease in its stability, which caused a loss of protein function. This study could be helpful to the genetic diagnosis of this disease.
Similar content being viewed by others
Background
Intellectual developmental disorder and microcephaly with pontine and cerebellar hypoplasia (MICPCH) is an X-linked disorder that affects females and is characterized by severe intellectual disability, microcephaly, and variable degrees of pontocerebellar hypoplasia [1, 2]. Affected individuals have very poor psychomotor development, often without independent ambulation or speech, and axial hypotonia with or without hypertonia. Some affected individuals may have sensorineural hearing loss or eye abnormalities. The dysmorphic features of those affected by this condition include overall poor growth and severe microcephaly (− 3.5 to − 10 SD). This causes the development of distinct facial features, such as broad nasal bridge and tip, large ears, long philtrum, micrognathia, and hypertelorism [3, 4]. It has been reported that variants of CASK, ITPR1, MARCKS, and RELN are involved in the aetiology of MICPCH [5, 6]. CASK is an excellent candidate gene for microcephaly disproportionate pontine and cerebellar hypoplasia (MICPCH) (MIM# 300749) since this gene functions in neuronal development. CASK mutant mice have small brains, abnormal cranial shapes, and cleft palates [4, 6,7,8,9,10]. Intragenic variants of CASK have been found in more than 50 individuals with the MICPCH phenotype [5, 11].
The CASK gene is a member of the MAGUK protein family. It maps to Xp11.4 and encodes CASK. CASK is a multidomain scaffolding protein composed of 926 amino acids (Ensembl ID: ENST00000378163) that is located at both the postsynaptic membrane of central nervous synapses and within the nuclei of neurons. CASK is highly expressed in the foetal brain [8, 12, 13]. Due to its location on the X chromosome, the loss-of-function of CASK usually leads to the manifestation of MICPCH in females [3, 5]. Males affected by CASK variants usually show more severe symptoms than females. These genetic issues are usually fatal in the womb for male embryos [7, 14].
In the clinical screening for this study, we first examined the case of an 11-month-old female patient with general developmental delay, microcephaly, and cerebellar hypoplasia. Whole exome screening indicated that the patient had a novel heterozygous missense variant in the CASK gene at the location NM_003688.3: c.638T>G, p.L213R. In this study, we used bioinformatics methods to predict the pathogenicity and harm of this missense variant in CASK. We also examined the effects of the variant on the mRNA and protein expression of CASK and predicted the structure of the protein. This study found that there is no difference in mRNA expression levels. However, amino acid variants were found to cause downstream changes in the stability of the spatial structure of the protein, and these changes downregulate protein expression and cause a loss of protein function.
Case presentation
Clinical summary
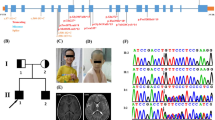
An unrelated natural couple brought an 11-month-old female with delayed development to the outpatient department for genetic counselling (Fig. 1a). There were no obvious abnormalities detected during the foetal period for this patient. After birth, the baby was found to have jaundice (lasting for 1 month), difficulties falling asleep and a small head circumference. At the age of 7 months, physical examination showed muscular hypertonia, hands often clenched, and global growth regression. The electroencephalogram showed bounded linearity. Cranial MRI showed that the volume of the bilateral cerebellar hemispheres was significantly decreased, especially in the lower part of cerebellar hemisphere, the cerebellar sulcus was widened and deepened, and the occipital cistern was widened (suspected cerebellar hemisphere dysplasia). Additionally, the signal of the bilateral globus pallidus changed slightly, the sulci of the bilateral cerebral hemispheres was slightly widened and deepened, the bilateral frontotemporal extracerebral space was slightly widened, and the bilateral lateral ventricles were slightly widened. At 11 months of age, the head circumference was 40 cm (< − 3 SD) (reference value: 41.9–47.3 cm) (Fig. 1b). The clinical diagnosis was primary microcephaly. There was no genetic history in the family, and the parents were not close relatives (Fig. 1c). We initially diagnosed the patient with intellectual developmental and microcephaly with pontine and cerebellar hypoplasia (MICPCH) based on the specific clinical characteristics. There were no phenotypic abnormalities observed in the parents of the patient.
Clinical summary for the patient. a Family pedigree. An unrelated natural couple who gave birth to the affected female. The black arrow denotes the proband. b The patient’s figure. c The patient’s axial brain MRI, which indicates the widening of the cerebral fissure and the shrinkage of the cerebellum. d PCR sequencing confirmed the CASK: NM_003688.3: exon 7: c.638T>G: p. L213R mutation in this family
Molecular genetic analysis
We extracted DNA from the patient’s peripheral blood sample and performed WES. The results showed that the CASK gene had a heterozygous missense variant, specifically CASK: NM_003688.3: exon 7: c.638T>G: p.L213R. (Fig. 1d) [15].
According to the bioinformatics analysis, this variant was not previous reported and was not found in most databases, including the ExAC browser, 1000 Genomes Project, and In-house Chinese-Control. The latest gnomAD database indicates that the frequency of this variant is 0.000005520 [16] (Table 1). In addition, this variant site is highly conserved in many species according to mutation taster (Fig. 2a). PhastCons and PhyloP were used to evaluate the scores of amino acid sequence conservation. The scores indicated that this variant site is highly conserved (Table 1). Moreover, this mutation was predicted to be deleterious by the following bioinformatic tools: SIFT [17], PolyPhen-2 [18], and M-CAP [19] (Table 1). The above results indicate that this variant site is pathogenic and well conserved.
Bioinformatic analysis of the conservation and pathogenicity of the variant in CASK. a Multiple sequence alignment of the CASK protein for different species. The black arrow denotes the position of the variant (c.638T>G: p.L213R). b The secondary and spatial structure prediction of the WT and MUT proteins
Pathogenicity analysis of the CASK variants
To further confirm the negative effect of this variant on CASK expression, wild-type and mutant plasmids were constructed and transfected into HEK-293T cells. We determined the mRNA (Fig. 3b) and protein expression (Fig. 3a, c) of both the wild type and the mutant and found that there was no significant difference in mRNA expression between the wild type and the mutant. However, compared with the wild type, the protein expression of the mutant was downregulated.
The negative effect of this variant on CASK expression. a Western blotting revealed a downregulation in the expression of the CASK protein after mutation. b qPCR revealed that there was no significant difference in the mRNA expression of CASK between the WT and MUT. c Immunofluorescence revealed a downregulation in the expression of the CASK protein after mutation
Finally, we predicted the structural pattern of the protein after the amino acid arginine (R) was substituted for leucine (L) by PSIPRED [20,21,22]. Importantly, the mutant protein showed decreased protein stability, which is represented by the increased Gibbs free energy (ΔΔGpred = 1.857). The results of the protein structure prediction showed that the nuclear charge of the protein increased (ΔCharge = 1) and the stability of the protein decreased (ΔΔGpred = 1.857) after the variant. Moreover, a random coil in the secondary structure is changed to a β-sheet, which also affects its spatial structure (Fig. 2b). Therefore, the decreased protein stability and the changed protein structure might contribute to the downregulation of CASK protein expression, further causing the loss of protein function.
Discussion and conclusions
In this study, we examined the case of a female with a novel heterozygous pathogenic missense variant in CASK that is associated with MICPCH. Moreover, the MRI results revealed a decreased size in both cerebellar hemispheres, a widening of the sulci in both cerebral hemispheres, and a widening and deepening of the cerebellar sulci. The WES results showed that the CASK gene had a heterozygous missense variant: NM_003688.3: exon 7: c.638T>G: p.L213R. Although there was no significant difference in the expression of CASK at the mRNA level between the wild type and mutant genes, the protein expression of CASK was downregulated. By using the protein structure prediction method, we found that when leucine was mutated to arginine in the primary sequence of the protein, the protein stability was reduced. Additionally, the secondary structure and spatial structure were changed. This resulted in reduced protein expression and the loss of protein function.
All residues in the αF helix have been shown to be involved in a conserved spatial pattern. Kornev et al. [23] suggested that the residue at the position equivalent to 209 in CASK plays a critical role in the anchoring of the αH helix, which is purported to help stabilize the hydrophobic core around the αF helix [12]. Similarly, in this study, the leucine at position 213 in the amino acid sequence of the CASK protein was mutated to arginine. Arginine is a basic amino acid and leucine is a nonpolar amino acid [24]. Thus, this amino acid change alters the tertiary structure of the protein [25], which probably impedes the anchoring of the αH helix. Furthermore, the destabilizing αH helix may not stabilize the hydrophobic core around αF, which may greatly disrupt the function of CASK as a protein kinase.
Generally, the types of variants that cause MICPCH are nonsense mutations, splice site mutations, frameshift mutations, and missense mutations. These types of variants usually result in a decrease or absence in CASK protein expression. The loss of CASK expression is associated with a more severe MICPCH phenotype and likely causes reduced viability or in utero lethality [7]. Missense variants in the CASK gene result in a mild intellectual developmental disorder that sometimes includes nystagmus, most often in males [26]. Our study also reports a missense variant, but the phenotype caused by this missense variant is more severe than that reported in a previous patient with a missense variant in CASK. This might be due to differences in the genetic backgrounds. Moreover, we found that this missense variant could change the properties of the amino acid sequence and changes the protein structure and stability of the CASK protein. These changes may lead to the downregulation of protein expression, further causing the loss of protein function.
In conclusion, this study reports a novel heterozygous pathogenic missense variant in CASK, which expands the spectrum of genetic alterations that cause CASK mutations. Moreover, this research is of great significance for understanding the pathogenicity of CASK point variants. This study further reveals the key role of CASK in MICPCH development and helps provide genetic explanations for the aetiology of CASK variants in MICPCH-affected patients.
Availability of data and materials
All data generated or analysed in this study are included in this published article and its Additional file 1. The raw datasets used and analysed during the current study are not deposited in publicly available repositories because of considerations about the security of human genetic resources. For other details of the availability of data and material, please refer to the methods section of the article and tables. Sequencing dataset can be obtained from the corresponding author on reasonable request.
Abbreviations
- MICPCH:
-
Intellectual developmental disorder and microcephaly with pontine and cerebellar hypoplasia
- FGS4:
-
FG Syndrome 4
- MRI:
-
Magnetic resonance imaging
- WES:
-
Whole-exome sequencing
References
LaConte LEW, Chavan V, Elias AF, Hudson C, Schwanke C, Styren K, Shoof J, Kok F, Srivastava S, Mukherjee K. Two microcephaly-associated novel missense mutations in CASK specifically disrupt the CASK–neurexin interaction. Hum Genet. 2018;137(3):231–46.
Cristofoli F, Devriendt K, Davis EE, Van Esch H, Vermeesch JR. Novel CASK mutations in cases with syndromic microcephaly. Hum Mutat. 2018;39(7):993–1001.
Moog U, Kutsche K, Kortum F, Chilian B, Bierhals T, Apeshiotis N, Balg S, Chassaing N, Coubes C, Das S, et al. Phenotypic spectrum associated with CASK loss-of-function mutations. J Med Genet. 2011;48(11):741–51.
Saitsu H, Kato M, Osaka H, Moriyama N, Horita H, Nishiyama K, Yoneda Y, Kondo Y, Tsurusaki Y, Doi H, et al. CASK aberrations in male patients with Ohtahara syndrome and cerebellar hypoplasia. Epilepsia. 2012;53(8):1441–9.
Hayashi S, Uehara DT, Tanimoto K, Mizuno S, Chinen Y, Fukumura S, Takanashi JI, Osaka H, Okamoto N, Inazawa J. Comprehensive investigation of CASK mutations and other genetic etiologies in 41 patients with intellectual disability and microcephaly with pontine and cerebellar hypoplasia (MICPCH). PLoS ONE. 2017;12(8): e0181791.
Hayashi S, Okamoto N, Chinen Y, Takanashi J, Makita Y, Hata A, Imoto I, Inazawa J. Novel intragenic duplications and mutations of CASK in patients with mental retardation and microcephaly with pontine and cerebellar hypoplasia (MICPCH). Hum Genet. 2012;131(1):99–110.
Najm J, Horn D, Wimplinger I, Golden JA, Chizhikov VV, Sudi J, Christian SL, Ullmann R, Kuechler A, Haas CA, et al. Mutations of CASK cause an X-linked brain malformation phenotype with microcephaly and hypoplasia of the brainstem and cerebellum. Nat Genet. 2008;40(9):1065–7.
Froyen G, Van Esch H, Bauters M, Hollanders K, Frints SG, Vermeesch JR, Devriendt K, Fryns JP, Marynen P. Detection of genomic copy number changes in patients with idiopathic mental retardation by high-resolution X-array-CGH: important role for increased gene dosage of XLMR genes. Hum Mutat. 2007;28(10):1034–42.
Becker M, Mastropasqua F, Reising JP, Maier S, Ho ML, Rabkina I, Li D, Neufeld J, Ballenberger L, Myers L, et al. Presynaptic dysfunction in CASK-related neurodevelopmental disorders. Transl Psychiatry. 2020;10(1):312.
Atasoy D, Schoch S, Ho A, Nadasy KA, Liu X, Zhang W, Mukherjee K, Nosyreva ED, Fernandez-Chacon R, Missler M, et al. Deletion of CASK in mice is lethal and impairs synaptic function. Proc Natl Acad Sci USA. 2007;104(7):2525–30.
Burglen L, Chantot-Bastaraud S, Garel C, Milh M, Touraine R, Zanni G, Petit F, Afenjar A, Goizet C, Barresi S, et al. Spectrum of pontocerebellar hypoplasia in 13 girls and boys with CASK mutations: confirmation of a recognizable phenotype and first description of a male mosaic patient. Orphanet J Rare Dis. 2012;7:18.
LaConte LEW, Chavan V, DeLuca S, Rubin K, Malc J, Berry S, Gail Summers C, Mukherjee K. An N-terminal heterozygous missense CASK mutation is associated with microcephaly and bilateral retinal dystrophy plus optic nerve atrophy. Am J Med Genet A. 2019;179(1):94–103.
Cohen AR, Woods DF, Marfatia SM, Walther Z, Chishti AH, Anderson JM. Human CASK/LIN-2 binds syndecan-2 and protein 4.1 and localizes to the basolateral membrane of epithelial cells. J Cell Biol. 1998;142(1):129–38.
Moog U, Bierhals T, Brand K, Bautsch J, Biskup S, Brune T, Denecke J, de Die-Smulders CE, Evers C, Hempel M, et al. Phenotypic and molecular insights into CASK-related disorders in males. Orphanet J Rare Dis. 2015;10:44.
Richards S, Aziz N, Bale S, Bick D, Das S, Gastier-Foster J, Grody WW, Hegde M, Lyon E, Spector E, et al. Standards and guidelines for the interpretation of sequence variants: a joint consensus recommendation of the American College of Medical Genetics and Genomics and the Association for Molecular Pathology. Genet Med. 2015;17(5):405–24.
Kearney HM, Thorland EC, Brown KK, Quintero-Rivera F, South ST. American College of Medical Genetics standards and guidelines for interpretation and reporting of postnatal constitutional copy number variants. Genet Med. 2011;13(7):680–5.
Kumar P, Henikoff S, Ng PC. Predicting the effects of coding non-synonymous variants on protein function using the SIFT algorithm. Nat Protoc. 2009;4(7):1073–81.
Adzhubei IA, Schmidt S, Peshkin L, Ramensky VE, Gerasimova A, Bork P, Kondrashov AS, Sunyaev SR. A method and server for predicting damaging missense mutations. Nat Methods. 2010;7(4):248–9.
Jagadeesh KA, Wenger AM, Berger MJ, Guturu H, Stenson PD, Cooper DN, Bernstein JA, Bejerano G. M-CAP eliminates a majority of variants of uncertain significance in clinical exomes at high sensitivity. Nat Genet. 2016;48(12):1581–6.
McGuffin LJ, Bryson K, Jones DT. The PSIPRED protein structure prediction server. Bioinformatics (Oxford, England). 2000;16(4):404–5.
Buchan DWA, Jones DT. The PSIPRED protein analysis workbench: 20 years on. Nucleic Acids Res. 2019;47(W1):W402-w407.
Buchan DW, Minneci F, Nugent TC, Bryson K, Jones DT. Scalable web services for the PSIPRED protein analysis workbench. Nucleic Acids Res. 2013;41(Web Server issue):W349–57.
Kornev AP, Taylor SS, Ten Eyck LF. A helix scaffold for the assembly of active protein kinases. Proc Natl Acad Sci USA. 2008;105(38):14377–82.
Grantham R. Amino acid difference formula to help explain protein evolution. Science (New York, NY). 1974;185(4154):862–4.
Vitkup D, Sander C, Church GM. The amino-acid mutational spectrum of human genetic disease. Genome Biol. 2003;4(11):R72.
Tarpey PS, Smith R, Pleasance E, Whibley A, Edkins S, Hardy C, O’Meara S, Latimer C, Dicks E, Menzies A, et al. A systematic, large-scale resequencing screen of X-chromosome coding exons in mental retardation. Nat Genet. 2009;41(5):535–43.
Acknowledgements
We thank the patient who provided samples for this research.
Funding
Not applicable.
Author information
Authors and Affiliations
Contributions
SW, CJ, JW and YS designed the experiments and conceptualized the study. SW and JL analysed the data. SW, GZ, JW and YS performed the experiments and wrote the manuscript. JW and YS supervised and approved the final draft of the document. All authors have read and agreed to the published version of the manuscript.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
Written informed consent to participate was obtained from the parents of the participant, who was under the age of 16, and the Medical Ethics Committee of West China Second Hospital of Sichuan University approved this procedure.
Consent for publication
Written informed consent for the publication of identifying images or other personal or clinical details was obtained from the parents of the participant, who was under the age of 18. We have included it as an Additional file 1.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1.
Methods and Materials in this study.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Wu, S., Jiang, C., Li, J. et al. A novel missense variant in the CASK gene causes intellectual developmental disorder and microcephaly with pontine and cerebellar hypoplasia. BMC Med Genomics 15, 127 (2022). https://doi.org/10.1186/s12920-022-01275-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12920-022-01275-z