Abstract
Fumaric acid is a valuable compound used in foods, beverages, detergents, animal feed, pharmaceuticals and miscellaneous industrial products. It is produced on a large scale by the petrochemical route but the current tendency is towards implementing “green production” and environmental friendly technologies like biotechnological production of fumaric acid using low-cost raw materials. In this context, numerous studies focus on improving the fermentation process not only by using renewable raw material and genetically modified microorganisms, but also by developing and applying different downstream techniques for easy recovery of fumaric acid from the fermented broth. This review presents the main methods for production and separation of fumaric acid, highlighting the advantages and disadvantages of these and the potential economic impact in industry.
Similar content being viewed by others
Introduction
Fumaric acid is a naturally occurring organic acid, an intermediate in the citric acid cycle and it is widely found in nature, being isolated for the first time from the plant Fumaria officinalis. This four-carbon dicarboxylic acid (Fig. 1), the trans-isomer of butenedioic acid, has many potential industrial applications, from the manufacture of chemical products (synthetic resins, biodegradable polymers) to food and pharmaceutical products (additive, therapeutic drugs) (Cao et al. 1996; Li et al. 2018; Tatara et al. 2017; Mrowietz et al. 2018).
Diverse applications of fumaric acid have led to its continuous increase production. Thus, in 1959 the estimated production of fumaric acid was about 4.53 kt most of which was utilized by the plastic industry for polyester resins, in 2012 the global fumaric acid market was estimated as 225.2 kt and in 2020 it is expected to be over 300 kt (Rhodes et al. 1961; Papadaki et al. 2017; Grand View 2015).
Nowadays, fumaric acid is produced by thermal or catalytic irreversible isomerization from maleic acid obtained from its anhydride by heating in presence of water (Das et al. 2016). An alternative process starts from n-butane, using vanadyl pyrophosphate (VO)2P2O5 as a catalyst (Cavani et al. 2010). Although petrochemical routes are considered as general recipes from the economic point of view, they are based on non-renewable resources and there is an increasing concern over sustainability. In this context, the utilization of microbial strains for fumaric acid production has been appraised, with the aim of its biotechnological production instead of the chemical route (Li et al. 2014; Dorsam et al. 2017; Deng and Aita 2018).
As for other compounds obtained by biological processes, the low-costs production for fumaric acid at industrial scale is triggered by the upstream stage (e.g. feedstock and microorganisms costs) and more importantly by the costs associated with downstream processing involved in the extraction of pure products from such systems. In this context, numerous studies focus on improving the fermentation process not only by using renewable raw material and genetically modified microorganisms, but also by developing and applying different downstream techniques for easy recovery of fumaric acid from the fermented broth (Prochaska et al. 2014; Gemici et al. 2015).
Applications
Commercial applications of fumaric acid are related to its chemical structure with a trans-double bond and two carboxylic acid groups, which makes from fumaric acid an attractive compound to be used in foods, beverages, detergents, animal feed, pharmaceuticals and miscellaneous industrial products, including lubricating oils, inks, lacquers, biodegradable polymers, and plasticizers (Table 1 with the references therein).
Demographic mobility and the changes in nutritional habits have made from the use of fumaric acid in food and farming industry the most important application this being accounted for 40% of world consumption (Markit 2018). Fumaric acid has been used in the food and farming industry as a nutritional additive, acidulant, flavoring and antimicrobial agent. It is currently used in wheat and corn tortillas, sour dough and rye breads, refrigerated biscuit doughs, fruit juice and nutraceutical drinks, gelatin desserts, gelling aids, pie fillings and wine. Due to its low molecular weight, fumaric acid has more buffering capacity than other food acids at pH near 3.0. It is a substitute for tartaric and citric acid, at a rate of 1.36 g of citric acid to every 0.91 grams of fumaric acid for the same taste thus reducing costs per unit weight. Also, fumaric acid can be used as an intermediate product in the preparation of other organic acids, such as l-malic acid and l-aspartic acid which are ingredients in food, health and cosmetic products (Das et al. 2016; Shimizu 2017; Deng and Aita 2018).
Supplementation with fumaric acid of ruminants’ diet has led to improved feed efficiency in ruminants by increasing propionate production and decreasing the hydrogen available for methanogenesis. Fumaric acid has also been successfully tested as an alternative to conventional antibiotic growth promoters and showed significant improvement in the feed-to-gain ratio in dairy and poultry industries (Deng and Aita 2018; Li et al. 2018).
Second, in terms of consumption, is production of unsaturated polyester resins (19% of world consumption) sustained by increased construction activity and the highly use in aerospace, automobile and tank/pipes production (Farmer et al. 2015; Markit 2018). The use of fumaric acid for production of rosin paper sizes acquires 18% of world consumption and it is one of the oldest and important applications of fumaric acid. However, the market of this application may decrease in the near future due to implementation of alkaline papermaking technology as a substitute (Markit 2018; Grand View 2015).
Newer applications of fumaric acid, and in particular, its esters (methyl, ethyl, propylfumarates), are implemented in the field of neurology, immunology, dermatology and bio-nanotechnology (drug delivery and tissue engineering). The US Food and Drug Administration has approved the fumaric acid use for the synthesis of ferrous fumarate prescribed for treatment of iron deficiency anemia and its ester, dimethyl fumarate was approved for the treatment of human adults with relapsing forms of multiple sclerosis (Mrowietz et al. 2018; fda.gov). Also, in vitro studies showed that fumaric acid esters promote neuronal survival upon ischemia and attenuate secondary degeneration after spinal cord injury highlighting the broad applications of fumaric acid esters in the medical field (Lin-Holderer et al. 2016; Mrowietz et al. 2018).
There is evidence that fumaric acid esters are not only effective and safe in psoriasis, but also in granulomatous non-infectious diseases like granuloma annulare, necrobiosis lipoidica, alopecia areata and sarcoidosis (Mrowietz et al. 2018).
Regarding tissue engineering, research groups focused on developing hydrogels based on fumaric acid, which are a group of synthetic biopolymers with distinct physical and mechanical properties, and each can be tailored to promote repair in response to the specific requirements of the damaged tissue (). For example, poly(propylene fumarate) can be subjected to crosslinking with a vinyl monomer, because of the presence of a double bond of the fumarate or it can be used to obtain poly(propylene fumarate)-co-poly(lactic-co-glycolic acid) scaffolds that can be used in bone repair surgery or cardiovascular applications. Biomedical field will benefit from the use of porous poly(propylene fumarate) scaffolds as drug delivery systems, magnetic resonance imaging—direct implantation, biosensors or neural interfaces, (Wu et al. 2018; Tatara et al. 2017).
Production
Currently, fumaric acid is primarily produced in two ways, the petrochemical route using different hydrocarbons as raw materials (e.g., benzene, n-butane or n-butane/n–butene mixture) and the conversion of biomass by microorganisms. Earlier methods for industrial production of fumaric acid were based on fungi fermentation, but it was discontinued as chemical synthesis became economically more attractive (Engel et al. 2008). Nowadays, with the depletion of petroleum sources and the increase awareness on the environmental pollution, many companies are conducting intense research in order to implement the biochemical route for fumaric acid production.
Fumaric acid production by petrochemical routes
Chemical synthesis of fumaric acid is the main commercially available method with a production of more than 90,000 tones/year. This method is based on the conversion of maleic anhydride to maleic acid and the cis–trans isomerization of maleic acid to fumaric acid. Benzene used to be the major starting material, but oxidation of n-butane or n-butane and n-butene mixtures has become more important in recent years. In this process, a small amount of water, formed as by-product can be directly liquefied from the reaction gas by partial condensation. The next step is the subjection of the mixture solvent-anhydride to fractional distillation to separate maleic anhydride from the solvent, the latter being returned to the absorption column. By thermal or catalytic cis–trans isomerization, maleic acid is converted almost quantitatively to fumaric acid. Further, the crude fumaric acid is purified by crystallization from water (Das et al. 2016; Shimizu 2017).
The conversion of maleic acid to fumaric acid requires the presence of homogenous or heterogeneous catalysts (inorganic acids, thiurea silica, zeolites, etc.) and even if the heterogeneous catalysts overcome some disadvantages presented by homogenous one (difficult to separate and reuse, high environmental pollution) their use at the industrial scale is limited due to lower selection and stability (Pritchard et al. 2015). In this context, the possibility to convert maleic acid to fumaric acid without catalysts was investigated by Gao et al. under high temperature and pressure conditions. By analyzing the reaction conditions the authors established the optimal conditions for isomerization of maleic acid to fumaric acid without catalysts. Thus, the maximum yield of fumaric acid (86%) was reached by using a concentration of 80% maleic acid, reaction temperature of 190 °C and reaction time 1 h (Gao et al. 2018).
Because the raw materials originate from petroleum, this method has a major disadvantage due to by-products of the processes which contaminate the environment. Even in the light of no catalysts isomerization of maleic acid, the petrochemical route requires expensive raw materials and high energy consumption. An alternative method, environmental friendly, could be the biotechnological production of fumaric acid using low-cost raw materials as a source of carbon, like apple juice, lignocellulose, food waste, glycerol (Li et al. 2014; Liu et al. 2016; Jimenez-Quero et al. 2016; Dorsam et al. 2017).
Enzymatic and microbial production of fumaric acid
Chemical production of fumaric acid by the isomerization of maleic acid is restricted by reaction equilibrium, the conversion yield being affected by the byproducts. An alternative method to produce fumaric acid from maleic acid was bioconversion using maleate isomerase. This enzyme is produced by microorganisms from Pseudomonas and Arthrobacter species. However, the maleate isomerase produced by Pseudomonas and Arthrobacter species is unstable even at moderate temperature which makes the conversion process more difficult. Studying this aspect, Goto et al. (1998) have shown that the microorganisms like Bacillus stearothermophilus, Bacillus brevis and Bacillus sp. produce thermo-stable maleate isomerase which can improve fumaric acid production process. Also, another way to improve the productivity and to reduce the production cost is to use the whole cell catalysis instead of cell-free enzymes (Goto et al. 1998).
In order to produce fumaric acid via bio-based malic acid enzymatic transformation, fumarase from Thermus thermophilus, an enzyme with high activity and stability, was overexpressed in Escherichia Coli and the influence of solvent nature (methanol, ethylene glycol, tert-butanol) and concentration (30–90%) was analyzed. The highest conversion rate, 54.7%, was obtained when using 50% ethylene glycol, at this solvent concentration the enzyme also showed a good stability (Liu et al. 2015).
Fermentative production of fumaric acid has been studied using three major classes of microorganisms: bacteria, yeast and fungi, the most employed being filamentous fungi from Rhizopus species (Table 2 with the references therein).
Production of fumaric acid has been studied using different Rhizopus species (R. nigricans, R. formosa, R. arrhizus, and R. Oryzae) and diverse carbon sources under both, aerobic and anaerobic conditions in order to establish the optimal parameters for biosynthesis of this acid. Generally, R. oryzae and R. arrhizus were preferred over the other Rhizopus species due to their simple nutrient requirements, the possibility to perform fermentation under aerobic and anaerobic conditions and adequate fumaric acid yield (Liu et al. 2017; Deng and Aita 2018).
High yields of fumaric acid can be usually obtained by two phase fermentation: (1) the seed culture phase in which the fungal growth is promoted by supplying the proper concentration and mixture of nutrients in the broth and (2) the acid production phase when the fungal growth is limited by decreasing the nitrogen concentration in the broth in order to promote the overproduction of fumaric acid (Deng and Aita 2018).
The nature of carbon and sources as well as the ratio between this two main components control the fungal morphology and the efficiency of fumaric acid production. The main carbon source is glucose, this being preferred because it is readily metabolized by fungi. In the current tendency towards using inexpensive renewable raw materials in the production of different compounds, several substrates were studied for fumaric acid fermentation. Thus alternative carbon sources include xylose, starch, lignocellulosic biomass, glycerol, and liquid food waste (Li et al. 2014; Wang et al. 2015; Wei et al. 2015; Liu et al. 2016; Dorsam et al. 2017). Renewable nitrogen sources have been analyzed too, to find an alternative to traditional nitrogen sources (ammonium salts, yeast extract, etc.). These alternative renewable nitrogen sources are corn step liquor, wheat bran or soybean cake hydrolysate (Wang et al. 2015; Papadaki et al. 2017).
The efficiency of a biosynthesis process is influenced by the following aspects: fungal morphology, medium composition, substrate selection, neutralizing agent, inoculum size, pH and culture rheology (Rhodes et al. 1961; Zhang et al. 2013).
The fungal morphology of Rhizopus spp. is influenced by various factors like pH, temperature, inoculum size, agitation and working volume. The formation of different fungal morphologies like clumps, filamentous mycelia or pellets during fermentation is affected by the growth conditions (Fig. 2) (Zhang et al. 2013). Small pellets are the preferred morphology for fumaric acid fermentation to reach optimum oxygen and mass transfer, these parameters being enhanced due to lower medium viscosity. However, depending on the fungi strain higher yields of fumaric acid can be achieved when using dispersed mycelia of R. arrhizus compared with pelletized biomass, while the opposite effect been reported in the case of R. delemar NRRL 1526. During fermentation, the disadvantage of filamentous or clumps morphologies comes from the tendency to grow on bioreactor wall and cause low oxygen transfer rates (Papadaki et al. 2017).
Morphologies of R. oryzae obtained under different culture conditions (Zhang et al. 2013)
At the industrial scale the fermentation with Rhizopus species can be limited mainly due to growing difficulties related to their morphology complex morphology causing difficulties in mixing and aeration. The use of immobilized biomass could be a good alternative because immobilization assures a constant morphology, protects microorganisms from pH or product inhibition, helps to purify final products, reduce the process time and enables production for multiple cycles (Gu et al. 2013; Liu et al. 2017; Naude and Nicol 2017). Thus, by using R. arrhizus RH-07-13 immobilized in a dyeing chemical fiber net the fermentation time was reduced with 83.3% compared to free-cell fermentation, while fumaric acid yield was similar (32.03 vs. 31.23 gL−1) (Gu et al. 2013).
For improving the efficiency of fumaric acid production by biotechnological processes different genetic techniques (high energy irradiations, use of mutagens or directed evolution) can be used to design microorganisms able to provide higher production yields (Li et al. 2014; Wei et al. 2015; Liu et al. 2017).
In the last years, significant progress has been made in exploring metabolic engineering pathways for biotechnological production of carboxylic acids as fumaric acid or malic acid using the yeast Saccharomyces cerevisiae. Reductive or oxidative routes can be applied to produce the dicarboxylic acids from tricarboxylic acid cycle (malic, fumaric, succinic acids), the reductive route being considered the best one (Song et al. 2013). One of the metabolic strategies used for fumaric acid production using S. cerevisiae is via reductive tricarboxylic acid cycle, which provides a maximum theoretical yield of 2 mol of fumaric acid per mole of glucose. The major challenge when using the yeast for fumaric acid production is driven from the fact that naturally the yeast cell cannot accumulate large quantities of fumaric acid in the cytosol because the cytosolic fumarase mainly catalyzes the conversion of fumaric acid to l-malic acid.
The construction of genetically engineered strains has also been considered as a mechanism for fumaric acid production. Thus, by using engineered strain of S. cerevisiae CEN.PK2-1CDTHI2 and glucose substrate, simultaneous reductive and oxidative routes were applied and the highest yield of fumaric acid (5.64 gL−1) was achieved by overexpression of pyruvate carboxylase and addition of biotin (Xu et al. 2013). Furthermore, yeast able to use substrate from renewable sources can be engineered to produce fumaric acid. In this aspect, Wei et al. engineered Scheffersomyces stipitis, the yeast with the highest native capacity for xylose fermentation, for fumaric acid production from xylose with the heterologous reductive pathway (Wei et al. 2015).
As an alternative way to obtain fumaric acid using microorganisms other than Rhizopus spp. Escherichia coli was designed to produce fumaric acid from different substrates as glucose or glycerol (Song et al. 2013; Li et al. 2014).
Methods for separation and quantification of fumaric acid
The downstream process is usually the main drawback of organic acids production since it involves multiple steps and requires high amount of materials and energy. In this context, many research groups studied different separation and purification methods for fumaric acid obtained by chemical or biological conversion. In the following, some methods for separation of fumaric acid are briefly presented.
Nonvolatile carboxylic acids, like fumaric acid, citric and malic acid, have been recovered and purified with several separation processes, including extraction, solvent extraction, ion exchange adsorption, precipitation or electrodialysis (Wozniak and Prochaska 2014) (Table 3 and the references therein). From the literature, it can be found that a lot of research has been carried out on using ion exchange adsorption and electrodialysis to separate carboxylic acids from fermentation broth, but these methods are not widely used in industry.
Carboxylic acids produced by fermentation, including fumaric acid can be separated at industrial scale with an in situ recovery method for simultaneous fermentation and separation (SFS). Through in situ process, enhanced product yield, productivity and titer can be achieved due to some valuable features of the process: avoidance of the metabolic shift due to overproduction of the end product or ease in pH control without addition of neutralizing agent. In a study using an SFS process in rotary biofilm contactor (RBC) coupled with an adsorption column the authors obtained an increase of the product yield from 74 to 85% (w/w) from glucose and the productivity from 3.08 to 4.25 gL−1h−1 fumaric acid. The productivity in the RBC reactor was three times higher than that of an equivalent stirred tank reactor and the RBC could be utilized repetitively for 2 weeks without losing any biological activity (Cao et al. 1996).
A precipitation–adsorption process was developed to overcome some disadvantages and to improve the conventional process for fumaric acid separation from fermentation broth. Thus, fumaric acid adsorption with activated carbon followed with desorption by acetone gave a high recovery yield of 93%. Also, the cost of the process can be reduced by operating the crystallization process at room temperature and by reusing the recovered activated carbon and acetone in another adsorption–desorption process (Zhang et al. 2014).
Direct recovery of fumaric acid from spent sulfite liquor-based fermentation broth was performed by Figueira et al. (2017) using a two-stage precipitation and by applying process integration to minimize the generation of waste streams containing fumaric acid an yield of 81.4% fumaric acid was obtained (Figueira et al. 2017).
Ion-exchange can be used for primary recovery of aqueous dicarboxylic acids (fumaric, itaconic, succinic and malic acid) at neutral pH, where there is almost complete acid dissociation. By studying the equilibria of anion exchange of aqueous dicarboxylate anions (fumarate, itaconate, malate and succinate) with anions (Cl− and OH−) bound to quaternary ammonium compounds (Aliquat extractant and Dowex sorbent), López-Garzón et al. (2017) showed that the ion-exchange occurs to a larger extent with OH− than with Cl− as counterion. Also, the sorbents had a larger ion exchange capacity than the extractants (López-Garzón et al. 2017).
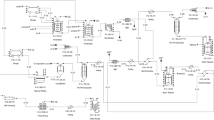
A hybrid system with nanofiltration, bipolar electrodialysis and reactive extraction was used to separate fumaric acid from fermentation broth resulted from the bioconversion of glycerol (Fig. 3). For the fermentation broth, containing succinic, acetic, fumaric and citric acids the authors showed that nanofiltration could be applied as one of the purification and concentration steps. As a result of bipolar electrodialysis of fermentation broth, a 61% recovery of fumaric acid was obtained and after applying a three steps reactive extraction 90% efficiency for fumaric acid recovery was recorded (Prochaska et al. 2014).
Hybrid system for separation of fumaric acid from fermentation broth (Prochaska et al. 2014)
Reactive extraction, a separation method used for recovery and purification of many biosynthetic compounds was applied to separate fumaric acid from its aqueous media by using tributyl amine (TBA) in different diluents (2-heptanone, 2-octanone, methyl isobutyl ketone, isoamyl alcohol, 1-octanol and 1-decanol). The highest extraction degree (96.29) was obtained using 2.080 molkg−1 TBA in isoamyl alcohol (Gemici et al. 2015). As reported in the literature, the extraction efficiency of acids depends on the type of extractant, diluents of the organic phase and the initial pH of the aqueous phase (Sood et al. 2014; Gemici et al. 2015).
Liquid membranes are defined as semi permeable phase separators and the traditional concept of membranes as polymer films can be extended to include liquids. This method is used as an alternative for reactive extraction or membrane electrodialysis because reduces the costs of the process for separation of different biosynthetic products (Galaction et al. 2015). Sood et al. (2014) investigated facilitated extraction of fumaric acid with an organic liquid membrane. The extraction system had the organic membrane consisted of toluene in which a carrier (4% tri-octylamine) was added and sodium hydroxide (1 N) was used as stripping phase. The experiments were performed with cell-free supernatant obtained from the fermentation of R. oryzae. The authors recorded the maximum extraction during the first 20 to 30 min of the reactive extraction run as fumaric acid concentration falls to almost 40% of its initial concentration after 30 min of extraction (Sood et al. 2014).
Extractive fermentation is used to separate carboxylic acids from the fermentation broth with an organic solvent. As organic solvents, long-chain aliphatic amines with high distribution coefficients are mostly used. This separating process is very attractive because it can produce and recover the desired acid product continuously in one step and reduce the end-product inhibition with significant increase of final product concentration. The extractive fermentation process could bring another promising way for economic and effective production of fumaric, citric, itaconic, and malic acids. The most common organic solvent used for this process is long-chain aliphatic amines with high distribution coefficients (Zhang et al. 2013).
Conclusions and future prospects
This work gives an overview of existing production and separation methods for fumaric acid, while further highlighting future tendencies. Fumaric acid is a valuable, multifunctional compound with uses ranging from food and beverages to construction and automotive. The large area of applications along with changes in nutritional habits and in environmental regulations represent the key motivation for developing new technology for production of fumaric acid, the market demand being forecast to increase by 6% per year during 2014–2020 (Grand View 2015).
Currently, the biochemical production of fumaric acid is less favorable economically than the petrochemical route due to lower productivity yield of the former one. The strain productivity, fermentation conditions, bioreactor design and downstream processing are the main aspects to be considered in order to optimize the biotechnological production of fumaric acid.
Genetically engineered microorganisms that are able to use different renewable materials, as carbon and nitrogen sources, could provide an economical alternative platform for production of fumaric acid.
Traditional downstream process of fumaric is complex, it involves many steps (acidification, heating, filtration, drying) and it induces high operational costs (Das et al. 2016; Figueira et al. 2017). As for other carboxylic acids, the reactive extraction using amines dissolved in non-toxic solvents, membrane technologies, nanofiltration and bipolar electrodialysis may represent advantageous methods for separation and purification of fumaric acid (Prochaska et al. 2014). Hybrid operation by merging various separation methods is used in industries in order to enhance the productivity, while reducing the separation steps and the process cost. Also, improving the conventional separation method based on precipitation and crystallization by manipulating the operational parameters or by using new compounds in the process may represent another way to increase the efficiency of the fumaric acid downstream processing.
Based on the above aspects, simultaneous fermentation and recovery may be an attractive alternative that could lead to high yield of fumaric acid with competitive price compared to chemical production route. Recovering the main product, fumaric acid, during fermentation allows a better control of pH fermentation and avoids the inhibition effect on the producing microorganisms.
References
Cao N, Du J, Gong CS, Tsao GT (1996) Simultaneous production and recovery of fumaric acid from immobilized Rhizopus oryzae with a rotary biofilm contactor and an adsorption column. Appl Environ Microbiol 62(8):2926–2931
Cavani F, Luciani S, Esposti ED, Cortelli C, Leanza R (2010) Surface dynamics of a vanadyl pyrophosphate catalyst for n-butane oxidation to maleic anhydride: an in situ Raman and reactivity study of the effect of the P/V atomic ratio. Chem A Eur J 16:1646–1655
Chen X, Wu J, Song W, Zhang L, Wang H, Liu L (2015) Fumaric acid production by Torulopsis glabrata: engineering the urea cycle and the purine nucleotide cycle. Biotechnol Bioeng 112(1):156–167
Das RK, Brar SK, Verma M (2016) Fumaric acid: production and application aspects. In: Brar SK, Sarma SJ, Pakshirajan K (eds) Platform chemical biorefinery: future green chemistry, edn. Elsevier, Amsterdam, pp 133–152
Deng F, Aita GM (2018) Fumaric acid production by Rhizopus oryzae ATCC®20344™ from lignocellulosic syrup. Bioenergy Res 11:330–340
Dorsam S, Fesseler J, Gorte O, Hahn T, Zibek S, Syldatk C, Ochsenreither K (2017) Sustainable carbon sources for microbial organic acid production with filamentous fungi. Biotechnol Biofuels 10(242):1–12
Engel CAR, Straathof AJJ, Zijlmans TW, van Gulik WM, van der Wielen LAM (2008) Fumaric acid production by fermentation. Appl Microbiol Biotechnol 78:379–389
Farmer TJ, Castle RL, Clark JH, Macquarrie DJ (2015) Synthesis of unsaturated polyester resins from various bio-derived platform molecules. Int J Mol Sci 16:14912–14932
Figueira D, Cavalheiro J, Ferreira BS (2017) Purification of polymer-grade fumaric acid from fermented spent sulfite liquor. Fermentation 3(13):1–11
Galaction AI, Poştaru M, Kloetzer L, Blaga AC, Caşcaval D (2015) Separation of rosmarinic acid by facilitated pertraction. Food Bioprod Process 94:621–628
Gao Z, Chen W, Chen X, Wang D, Shouzhi Y (2018) Study on the isomerization of maleic acid to fumaric acid without catalyst. Bull Korean Chem Soc 39(8):920–924
Gemici A, Uslu H, Kirbaşlar SI (2015) Extractability of fumaric acid by tributyl amine (TBA) in ketones and alcohols. J Chem Eng Data 60:2717–2720
Goto M, Nara T, Tokumaru I, Fugono N, Uchida Y, Terasawa M, Yukawa H (1998) Method of producing fumaric acid. US5783428
Grand View Research, Inc. (2015) Fumaric acid market analysis and segment forecasts to 2020, Report ID 978-1-68038-374-4, https://www.grandviewresearch.com/industry-analysis/fumaric-acid-market
Gu C, Zhou Y, Liu L, Tan T, Deng L (2013) Production of fumaric acid by immobilized Rhizopus arrhizus on net. Bioresour Technol 131:303–307
Jimenez-Quero A, Pollet E, Zhao M, Marchioni E, Avérous L, Phalip V (2016) Itaconic and fumaric acid production from biomass hydrolysates by Aspergillus Strains. J Microbiol Biotechnol 26(9):1557–1565
Li N, Zhang B, Wang Z, Tang YJ, Chen T, Zhao X (2014) Engineering Escherichia coli for fumaric acid production from glycerol. Bioresour Technol 174:81–87
Li Z, Liu N, Cao Y, Jin C, Li F, Cai C, Yao J (2018) Effects of fumaric acid supplementation on methane production and rumen fermentation in goats fed diets varying in forage and concentrate particle size. J Anim Sci Biotechnol 9(21):1–9
Lin-Holderer J, Li L, Gruneberg D, Marti HH, Kunze R (2016) Fumaric acid esters promote neuronal survival upon ischemic stress through activation of the Nrf2 but not HIF-1 signaling pathway. Neuropharmacology 105:228–240
Liu Y, Song J, Tan T, Liu L (2015) Production of fumaric acid from L-malic acid by solvent engineering using a recombinant thermostable fumarase from Thermus thermophilus HB8. Appl Biochem Biotechnol 175(6):2823–2831
Liu H, Ma J, Wang M, Wang W, Deng L, Nie K, Yue X, Wang F (2016) Food waste fermentation to fumaric acid by Rhizopus arrhizus RH7-13. Appl Biochem Biotechnol 180:1524–1533
Liu H, Zhao S, Jin Y, Yue X, Deng L, Wang F, Tan T (2017) Production of fumaric acid by immobilized Rhizopus arrhizus RH 7-13-9# on loofah fiber in a stirred-tank reactor. Bioresour Technol 244(Pt 1):929–933
López-Garzón CS, van der Wielen LAM, Straathof AJJ (2017) Strong anion exchange recovery of aqueous dicarboxylates: extraction and sorption equilibrium comparison. Sep Purif Technol 175:9–18
Markit I (2018) Fumaric Acid: Chemical Economics Handbook. https://ihsmarkit.com/fumaric. Accessed 5 Oct 2018
Mrowietz U, Morrison PJ, Suhrkamp I, Kumanova M, Clement B (2018) The Pharmacokinetics of fumaric acid esters reveal their in vivo effects. Trends Pharmacol Sci 39(1):1–12
Naude A, Nicol W (2017) Fumaric acid fermentation with immobilised Rhizopus oryzae: quantifying time-dependent variations in catabolic flux. Process Biochem 56:8–20
Papadaki A, Androutsopoulos N, Patsalou M et al (2017) Biotechnological production of fumaric acid: the effect of morphology of Rhizopus arrhizus NRRL 2582. Fermentation 3(33):1–13
Pritchard J, Filonenko GA, van Putten R, Hensen EJ, Pidko EA (2015) Heterogeneous and homogeneous catalysis for the hydrogenation of carboxylic acid derivatives: history, advances and future directions. Chem Soc Rev 44(11):3808–3833
Prochaska K, Staszak K, Wozniak-Budych MJ, Regel-Rosocka M, Adamczak M, Wisniewski M, Staniewski J (2014) Nanofiltration, bipolar electrodyalisis and reactive extraction hybrid system for separation of fumaric acid from fermentation broth. Bioresour Technol 167:219–225
Rhodes RA, Moyer AJ, Smith ML, Kelley SE (1961) Production of fumaric acid in 20 liter fermenters. Appl Microbiol 10(1):9–15
Shimizu K (2017) Metabolic engineering for the production of a variety of biofuels and biochemical. Metabolic regulation and metabolic engineering for biofuel and biochemical production. CRC Press, Boca Raton, pp 186–208
Song CW, Kim D, Choi S, Jang JW, Lee SY (2013) Metabolic engineering of Escherichia coli for the production of fumaric acid. Biotechnol Bioeng 110(7):2025–2034
Sood Y, Dhawan J, Kaur A (2014) Fumaric acid production by Rhizopus oryzae and facilitated extraction via organic liquid membrane. Afr J Biotechnol 13(10):1182–1187
Tatara AM, Watson E, Satish T, Scott TW, Kontoyiannis DP, Engel S, Mikos AG (2017) Synthesis and characterization of diol-based unsaturated polyesters: Poly(diol fumarate) and Poly(diol fumarate-co-succinate). Biomacromol 18:1724–1735
Wang G, Huang D, Li Y, Wen J, Jia X (2015) A metabolic-based approach to improve xylose utilization for fumaric acid production from acid pretreated wheat bran by Rhizopus oryzae. Bioresour Technol 180:119–127
Wei L, Liu J, Qi H, Wen J (2015) Engineering Scheffersomyces stipitis for fumaric acid production from xylose. Bioresour Technol 187:246–254
Wozniak MJ, Prochaska K (2014) Fumaric acid separation from fermentation broth using nanofiltration (NF) and bipolar electrodialysis (EDBM). Sep Purif Technol 125:179–186
Wu W, Liu X, Zhou Z, Miller AL, Lu L (2018) Three-dimensional porous poly(propylene fumarate)-co-poly(lactic-co-glycolic acid) scaffolds for tissue engineering. J Biomed Mater Res A 106(9):2507–2517
Xu G, Chen X, Liu L, Jiang L (2013) Fumaric acid production in Saccharomyces cerevisiae by simultaneous use of oxidative and reductive routes. Bioresour Technol 148:91–96
Zhang K, Zhang B, Yang HT (2013) Production of citric, itaconic, fumaric and malic acids in filamentous fungal fermentations. In: El-Enshasy HA, Thongchul N (eds) Bioprocessing technologies in biorefinery for sustainable production of fuels, chemicals, and polymers, 1st edn. Wiley, Hoboken, pp 375–397
Zhang K, Zhang L, Yang SY (2014) Fumaric acid recovery and purification from fermentation broth by activated carbon adsorption followed with desorption by acetone. Ind Eng Chem Res 53(32):12802–12808
Acknowledgements
This work was supported by the Grant PN-III-P4-ID-PCE-2016-0100 authorized by The National Council for Scientific Research - Executive Unit for Financing Higher Education, Research, Development and Innovation (CNCS-UEFISCDI).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Ilica, RA., Kloetzer, L., Galaction, AI. et al. Fumaric acid: production and separation. Biotechnol Lett 41, 47–57 (2019). https://doi.org/10.1007/s10529-018-2628-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10529-018-2628-y