Abstract
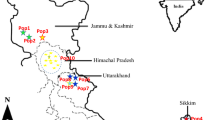
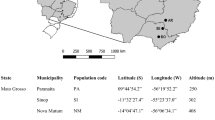
Rubia cordifolia L., is an industrially viable medicinal crop and is widely exploited for the therapeutic potential of its bioactive metabolite, purpurin. The present investigation aimed to explore the chemotypic and molecular variability in seven wild populations of R. cordifolia from South Eastern Ghats region of India. Thirty-eight individuals were assessed for molecular fingerprinting (ISSR markers) and densitometric quantification of purpurin and alizarin. The populations of Yelagiri Hills and Shervaroy Hills contained the highest levels of alizarin (0.115 and 0.093%, respectively) while Pachamalai and Kolli Hills revealed the highest purpurin content (0.284 and 0.280%, respectively). Genetic diversity was generally higher in the same populations that produced higher metabolite content, with the exception of Pachamalai, suggesting a highly prioritized conservation concern. The study revealed a Nei’s total gene diversity at species level of 0.266 and of 0.187 at population level, with an average population genetic differentiation of 0.28. No clear genetic or chemical structure was retrieved between the studied populations, with individuals from different locations clustering together, and no significant correlation was obtained between metabolites and genetic diversity or between these and the populations’ geographic distances.





Similar content being viewed by others
References
Baghalian K, Maghsodi M, Naghavi MR (2010) Genetic diversity of Iranian madder (Rubia tinctorum) populations based on agro-morphological traits, phytochemical content and RAPD markers. Ind Crops Prod 31:557–562
Bahulikar RA, Lagu MD, Kulkarni BG, Pandit SS, Suresh HS, Rao MKV, Ranjekar PK, Gupta VS (2004) Genetic diversity among spatially isolated populations of Eurya nitida Korth. (Theaceae) based on inter-simple sequence repeats. Curr Sci 86:824–831
Bhatt P, Kushwah AS (2013) Rubia cordifolia overview: a new approach to treat cardiac disorders. Int J Drug Dev Res 5:47–54
Bobby MDN, Wesely EG, Johnson M (2012) High performance thin layer chromatography profile studies on the alkaloids of Albizia lebbeck. Asian Pac J Trop Biomed 2:S1–S6
Bodare S, Tsuda Y, Ravikanth G, Shaanker UR, Lascoux M (2013) Genetic structure and demographic history of the endangered tree species Dysoxylum malabaricum (Meliaceae) in Western Ghats, India: implications for conservation in a biodiversity hotspot. Ecol Evol 3:3233–3248
Bornet B, Branchard M (2001) Non-anchored inter-simple sequence repeat (ISSR) markers: reproducible and specific tools for genome fingerprinting. Plant Mol Bio Rep 19:209–215
Cieslak E, Szelag Z (2010) Genetic diversity of Galium cracoviense, G. oelandicum and G. sudeticum (Rubiaceae)—narrow endemic species of Galium sect. Leptogalium in northeastern Europe. Acta Soc Bot Pol 79:269–275
da Silva AVC, Freire KCS, Ledo ADS, Rabbani ARC (2014) Diversity and genetic structure of jenipapo (Genipa americana L.) Brazilian accessions. Sci Agric 71:345–355
Daman R, Bhandari S, Singh B, Brij L, Pathania S (2006) Comparative Studies of Rubia cordifolia L. and its commercial samples. Ethnobot Leaflets 11:179–188
Deshkar N, Tilloo S, Pande V (2008) A comprehensive review of Rubia cordifolia. Linn Pharm Rev 2:124–134
Ersts PJ (2016) Geographic distance matrix generator (version 1.2.3). American Museum of Natural History, Centre for Biodiversity and Conservation. http://biodiversityinformatics.amnh.org/open_source/gdmg. Accessed 17 Mar 2016
Fecka I, Cisowski W (2002) TLC determination of tannins and flavonoids in extracts from some Erodium species using chemically modified stationary phases. J Planar Chromatogr 15:429–432
Felsenstein J (1985) Confidence limits on phylogenies: an approach using the bootstrap. Evolution 39:783–791
Ferran SR, Olga J, Isidre C, Cristina AL, Maria IP, Rosa LR (2003) Liquid chromatographic/electrospray ionization tandem mass spectrometric study of the phenolic composition of cocoa (Theobroma cacao). J Mass Spectrum 38:35–42
Gaafar ARZ, Al-Qurainy F, Khan S (2014) Assessment of genetic diversity in the endangered populations of Breonadia salicina (Rubiaceae) growing in The Kingdom of Saudi Arabia using inter-simple sequence repeat markers. BMC Genet 15:1–10
Garcia VS, Palau J, Romeu A (1999) Horizontal gene transfer in glycosyl hydrolases inferred from codon usage in Escherichia coli and Bacillus subtilis. Mol Bio Evol 16:1125–1134
Hamrick JL, Godt MJW (1996) Effects of life history traits on genetic diversity in plant species. Philos Trans R Soc Lond B Biol Sci 351:1291–1298
Jaccard P (1908) Nouvelle recherches sur La distribution florale. Bulletin de la Société vaudoise des sciences naturelles 44:223–270
Khanuja SPS, Shasany AK, Darokar MP, Kumar S (1999) Rapid isolation of DNA from dry and fresh samples of plants producing large amounts of secondary metabolites and essential oils. Plant Mol Bio Rep 17:1–7
Khare C (2004) Encyclopedia of Indian medicinal plants—Rational Western therapy, Ayurvedic and other traditional usage. Springer, Germany, ISBN: 3–540–2003, 3–9
Ku YR, Chen CY, Ho LK, Lini JH, Chang YS (2002) Analysis of flavonoids in Vernonia paltula by high-performance liquid chromatography. J Food Drug Anal 10:139–142
Leonard DS (2009) A visual approach to SPSS for windows: a guide to SPSS 17.0, Needham Heights. Allyn and Bacosn Inc, MA
Lewinton RC (1972) The apportionment of human diversity. Evol Biol 6:381–398
Li J, Jin Z (2007) Genetic variation and differentiation in Torreya jackii Chun, an endangered plant endemic to China. Plant Sci 172:1048–1053
Liu CS, Song YS, Zhang KJ, Ryu JC, Kim M, Zhou TU (1995) Gas chromatographic/mass spectrometric profiling of luteolin and its metabolites in rat urine and bile. J Pharm Biomed Anal 13:1409–1414
Liu FF, Catharina YWA, Heinze TM, Rankin JD, Berger RD, Freeman JP, Lay JO (2000) Evaluation of major active components in St. John’s Wort dietary supplements by high-performance liquid chromatography with photodiode array detection and electrospray mass spectrometric confirmation. J Chromatogr A 888:85–92
Mantel N (1967) The detection of disease clustering and a generalized regression approach. Cancer Res 27:209–220
Mishchenko NP, Fedoreev SA, Bryukhanov VM, Zverev YF, Lampatov VV, Azarova OV, Shkry YN, Chernoded GK (2007) Chemical composition and pharmacological activity of anthraquinones from R. cordifolia cell culture. Pharm Chem J 41:38–41
Mishra P, Kumar LD, Kumar GS, Ravikumar K, Shukla AK, Sundaresan V (2015) Population dynamics and conservation implications of Decalepis arayalpathra (J. Joseph and V. Chandras.) Venter., a steno endemic species of Western Ghats, India. Appl Biochem Biotechnol 176:1413–1430
Naik D, Singh D, Vartak V, Paranjpe S, Bhargava S (2009) Assessment of morphological and genetic diversity in Gmelina arborea Roxb. New For 38:99–115
Nei M (1973) Analysis of gene diversity in subdivided populations. PNAS 70:3321–3323
Nei M (1987) Molecular evolutionary genetics. Columbia University Press, New York
Panda S, Naik D, Kamble A (2015) Population structure and genetic diversity of the perennial medicinal shrub Plumbago. AoB Plants. 7:plv048. https://doi.org/10.1093/aobpla/plv048
Pandotra P, Gupta AP, Husain MK, Gandhiram Gupta S (2013) Evaluation of genetic diversity and chemical profile of ginger cultivars in north-western Himalayas. Biochem Sys Ecol 48:281–287
Peakall R, Smouse PE (2006) GenAlEx V 6: genetic analysis in Excel. Population genetic software for teaching and research. Mol Ecol Notes 6:288–295
Pereira CAM, Yariwake JH, Lanças FM, Wauters JN, Tits M, Angenot L (2004) A HPTLC denistometric determination of flavonoids from Passiflora alata, P. edulis, P. incarnata and P. caerulea and comparison with HPLC Method. Phytochem Anal 15:241–248
Pither R, Shore JS, Kellman M (2003) Genetic diversity of the tropical tree Terminalia amazonia (Combretaceae) in naturally fragmented populations. Heredity 91:307–313
Prasanth RK, Nandagopal S (2009) Assessment of genetic variation in Rubia cordifolia L. using isozyme data. Int J Appl Bioeng 4:38–40
Roldan-Ruiz I, Dendauw J, Vanbockstaele E, Depicker A, De-Loose M (2000) AFLP markers reveal high polymorphic rates in ryegrasses (Lolium spp.). Mol Breed 6:125–134
Sisubalan N, Amzad Basha K, Ghouse BM (2015) Assessment of alizarin, purpurin and genetic fidelity of Rubia Cordifolia L. from Eastern Ghats of Tamil Nadu. Int J Pharm Bio Sci 6:1112–1122
Snedecor GW, Cochran WG (1995) Statistical methods, 8th edn. Iowa State University Press, Ames, Iowa, p 30
Szepesi G, Nyiredy S (1992) Planar chromatography: current status and future perspectives in pharmaceutical analysis. I. Applicability, quantization and validation. J Pharm Biomed Anal 10:1007–1015
Van-De-Peer Y, Wachter R (1994) TREECON for Windows: a software package for the construction and drawing of evolutionary trees for the Microsoft Windows environment. Comput App Biosci 10:569–570
Ved D, Oommen S, Singh A (2002) Propagation and agro-technology status of commercially important medicinal plant species of the project area of Andhra Pradesh community forest management project. Foundation for Revitalization of Local Health Traditions (FRLHT), Andhra Pradesh Forest Department, Bangalore, p 128
Wang X, Yang R, Feng S, Hou X, Zhang Y, Li Y, Ren Y (2012) Genetic variation in Rheum palmatum and Rheum tanguticum (Polygonaceae), two medicinally and endemic species in China using ISSR markers. PLoS ONE 7e:51667
Xia T, Chen S, Chen S, Ge X (2005) Genetic variation within and among populations of Rhodiola alsia (Crassulaceae) native to the Tibetan Plateau as detected by ISSR markers. Biochem Genet 43:87–101
Yang W, de-Oliveira AC, Godwin I, Schertz K, Bennetzen JL (1996) Comparison of DNA marker technologies in characterizing plant genome diversity: variability in Chinese sorghums. Crop Sci 36:1669–1676
Yeh F, Yang R, Boyle T (1999) Microsoft window-based freeware for population genetic analysis (POPGENE Ver. 1.32). University of Alberta, Canada
Young A, Boyle T, Brown T (1996) The population genetic consequences of habitat fragmentation for plants. Trends Ecol Evol 11:413–418
Zhao W, Shi X, Li J, Guo W, Liu C (2014) Genetic, epigenetic, and HPLC fingerprint differentiation between natural and ex situ populations of Rhodiola sachalinensis from changbai mountain, China. PLoS ONE 9e:112869
Zietkiewiez E, Rafalski A, Labuda D (1994) Genome fingerprinting by simple sequence repeat (SSR)—anchored polymerase chain reaction amplification. Genomics 20:176–183
Acknowledgements
The authors wish to express their sincere thanks to Director, CSIR-CIMAP, Lucknow, India for providing the facilities to carry out this research (CIMAP Communication Number: CIMAP/PUB/2016/78). The financial support from the University Grants Commission (UGC), Government of India through Research Project (F.NO.39-368/2010) (SR) is gratefully acknowledged.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Natarajan, S., Mishra, P., Vadivel, M. et al. ISSR Characterization and Quantification of Purpurin and Alizarin in Rubia cordifolia L. Populations from India. Biochem Genet 57, 56–72 (2019). https://doi.org/10.1007/s10528-018-9875-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10528-018-9875-4