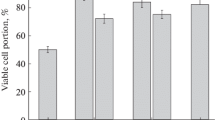
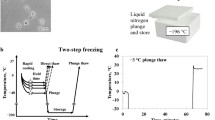
We studied the effect of storage conditions on the safety of microvesicles produced by human multipotent umbilical cord mesenchymal stromal cells into the conditioned medium. It was found that microvesicles can be stored without serious degradation for up to 1 week at 4°С, but were almost completely destroyed during freezing and thawing cycles irrespective of the storage temperatures (-20°С, -70°С, or -196°С). Similar results were obtained for lyophilized medium conditioned by human multipotent umbilical cord mesenchymal stromal cells. Addition of a cryoprotectant (5-10% DMSO) followed by freezing and/or lyophilization preserved microvesicles at a nearly initial level. These findings indicate that during storage, microvesicles, being membrane structures, behave similar to living cells and require appropriate conditions for prolonged storage.
Similar content being viewed by others
References
Romanov YA, Balashova EE, Bystrykh OA, Titkov KV, Dugina TN, Kabaeva NV, Fedorova TA, Rogachevskii OV, Degtyarev DN, Sukhikh GT. Umbilical cord blood for autologous transfusion in the early postnatal ontogeny: analysis of cell composition and viability during long-term culturing. Bull. Exp. Biol. Med. 2015;158(4):523-527.
Romanov YA, Balashova EE, Volgina NE, Kabaeva NV, Dugina TN, Sukhikh GT. Optimized Protocol for Isolation of Multipotent Mesenchymal Stromal Cells from Human Umbilical Cord. Bull. Exp. Biol. Med. 2015;160(1):148-154.
Romanov YA, Balashova EE, Volgina NE, Kabaeva NV, Dugina TN, Sukhikh GT. Isolation of Multipotent Mesenchymal Stromal Cells from Cryopreserved Human Umbilical Cord Tissue. Bull. Exp. Biol. Med. 2016;160(4):530-534.
Romanov YA, Balashova EE, Volgina NE, Kabaeva NV, Dugina TN, Sukhikh GT. Human Umbilical Cord Blood Serum: Effective Substitute of Fetal Bovine Serum for Culturing of Human Multipotent Mesenchymal Stromal Cells. Bull. Exp. Biol. Med. 2017;162(4):528-533.
Romanov YA, Balashova EE, Volgina NE, Kabaeva NV, Dugina TN, Sukhikh GT. Expression of Surface Molecules in Human Mesenchymal Stromal Cells Co-Cultured with Nucleated Umbilical Cord Blood Cells. Bull. Exp. Biol. Med. 2017;162(4):578-582.
Romanov YA, Volgina NE, Balashova EE, Kabaeva NV, Dugina TN, Sukhikh GT. Human Umbilical Cord Mesenchymal Stromal Cells Support Viability of Umbilical Cord Blood Hematopoietic Stem Cells but not the “Stemness” of Their Progeny in Co-Culture. Bull. Exp. Biol. Med. 2017;163(4):523-527.
Romanov YA, Volgina NE, Vtorushina VV, Romanov AY, Dugina TN, Kabaeva NV, Sukhikh GT. Comparative Analysis of Secretome of Human Umbilical Cord- and Bone Marrow-Derived Multipotent Mesenchymal Stromal Cells. Bull. Exp. Biol. Med. 2019;166(4):535-540.
Romanov YA, Volgina NE, Dugina TN, Kabaeva NV, Sukhikh GT. Human Umbilical Cord Mesenchymal Stromal Cell-Derived Microvesicles Express Surface Markers Identical to the Phenotype of Parental Cells. Bull. Exp. Biol. Med. 2018;166(1):124-129.
Romanov YuA, Romanov AYu. Tissues of perinatal origin: a unique source of cells for regenerative medicine. Part II. Umbilical cord. Neronatologiya. 2018;6(3):54-73.
Abbasi-Malati Z, Roushandeh A. M, Kuwahara Y, Roudkenar MH. Mesenchymal stem cells on horizon: a new arsenal of therapeutic agents. Stem Cell Rev. 2018;14(4):484-499.
Batsali AK, Kastrinaki MC, Papadaki HA, Pontikoglou C. Mesenchymal stem cells derived from Wharton’s Jelly of the umbilical cord: biological properties and emerging clinical applications. Curr. Stem Cell Res. Ther. 2013;8(2):144-155.
Beer L, Mildner M, Ankersmit HJ. Cell secretome based drug substances in regenerative medicine: when regulatory affairs meet basic science. Ann. Transl Med. 2017;5(7):170.
Can A, Celikkan FT, Cinar O. Umbilical cord mesenchymal stromal cell transplantations: A systemic analysis of clinical trials. Cytotherapy. 2017;19(12):1351-1382.
Caplan AI. Mesenchymal stem cells. J. Orthop. Res. 1991;9(5):641-650.
Ghaderi A, Abtahi S. Mesenchymal stem cells: miraculous healers or dormant killers? Stem Cell Rev. 2018;14(5):722-733.
Kalaszczynska I, Ferdyn K. Wharton’s jelly derived mesenchymal stem cells: future of regenerative medicine? Recent findings and clinical significance. Biomed. Res. Int. 2015;2015. ID 430847. doi: https://doi.org/10.1155/2015/430847.
Keshtkar S, Azarpira N, Ghahremani MH. Mesenchymal stem cell-derived extracellular vesicles: novel frontiers in regenerative medicine. Stem Cell Res. Ther. 2018;9(1):63. doi: https://doi.org/10.1186/s13287-018-0791-7.
Konala VB, Mamidi MK, Bhonde R, Das AK, Pochampally R, Pal R. The current landscape of the mesenchymal stromal cell secretome: A new paradigm for cell-free regeneration. Cytotherapy. 2016;18(1):13-24.
Pashoutan Sarvar D, Shamsasenjan K, Akbarzadehlaleh P. Mesenchymal stem cell-derived exosomes: new opportunity in cell-free therapy. Adv. Pharm. Bull. 2016;6(3):293-299.
Pawitan JA. Prospect of stem cell conditioned medium in regenerative medicine. Biomed. Res. Int. 2014;2014. ID 965849. doi: https://doi.org/10.1155/2014/965849.
Zhang B, Shen L, Shi H, Pan Z, Wu L, Yan Y, Zhang X, Mao F, Qian H, Xu W. Exosomes from human umbilical cord mesenchymal stem cells: identification, purification, and biological characteristics. Stem Cells Int. 2016;2016. ID 1929536. doi: https://doi.org/10.1155/2016/1929536.
Author information
Authors and Affiliations
Corresponding author
Additional information
Translated from Kletochnye Tekhnologii v Biologii i Meditsine, No. 1, pp. 12-16, January, 2019
Rights and permissions
About this article
Cite this article
Romanov, Y.A., Volgina, N.E., Dugina, T.N. et al. Effect of Storage Conditions on the Integrity of Human Umbilical Cord Mesenchymal Stromal Cell-Derived Microvesicles. Bull Exp Biol Med 167, 131–135 (2019). https://doi.org/10.1007/s10517-019-04476-2
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10517-019-04476-2