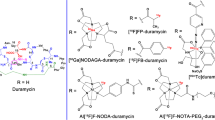
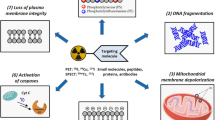
Abstract
Phosphatidylethanolamine (PE) is one of the most abundant phospholipids in mammalian plasma membranes. In healthy cells, PE resides predominantly in the inner leaflet of the cell membrane. In dead or dying cells on the other hand, PE is externalized to the outer leaflet of the plasma membrane. The exposure of PE on the cell surface has therefore become an attractive target for the molecular imaging of cell death using single-photon emission computed tomography (SPECT) and positron emission tomography (PET). This has motivated the development of PE-specific probes to measure cell death in vitro and non-invasively in vivo. In this review, we highlight the biological roles of PE on cell membranes, and PE exposure as a biomarker of cell death in disease processes, along with the use of PE-binding molecular probes to target PE for the characterization of cell death on a cellular and tissue level. We specifically emphasize the preclinical applications of radiolabeled duramycin for the non-invasive imaging of cell death in animal models of disease and in tumors after therapy. In addition, we discuss the clinical relevance, limitations and future perspectives of this imaging approach of cell death.




Adapted from reference [44]


Adapted from reference [71]

Adapted from reference [82]

Adapted from reference [89]

Adapted from reference [91]. (Color figure online)
Similar content being viewed by others
References
van Meer G, Voelker DR, Feigenson GW (2008) Membrane lipids: where they are and how they behave. Nat Rev Mol Cell Biol 9(2):112–124. doi:10.1038/nrm2330
Fadeel B, Xue D (2009) The ins and outs of phospholipid asymmetry in the plasma membrane: roles in health and disease. Crit Rev Biochem Mol Biol 44(5):264–277. doi:10.1080/10409230903193307
Orr JW, Newton AC (1992) Interaction of protein kinase C with phosphatidylserine. 2. Specificity and regulation. BioChemistry 31(19):4667–4673
Emoto K, Toyama-Sorimachi N, Karasuyama H, Inoue K, Umeda M (1997) Exposure of phosphatidylethanolamine on the surface of apoptotic cells. Exp Cell Res 232(2):430–434. doi:10.1006/excr.1997.3521
Zhao M, Li Z, Bugenhagen S (2008) 99mTc-labeled duramycin as a novel phosphatidylethanolamine-binding molecular probe. J Nucl Med 49(8):1345–1352. doi:10.2967/jnumed.107.048603
Blankenberg FG, Katsikis PD, Tait JF, Davis RE, Naumovski L, Ohtsuki K, Kopiwoda S, Abrams MJ, Darkes M, Robbins RC, Maecker HT, Strauss HW (1998) In vivo detection and imaging of phosphatidylserine expression during programmed cell death. Proc Natl Acad Sci USA 95(11):6349–6354
Benali K, Louedec L, Azzouna RB, Merceron O, Nassar P, Al Shoukr F, Petiet A, Barbato D, Michel JB, Sarda-Mantel L, Le Guludec D, Rouzet F (2014) Preclinical validation of 99mTc-annexin A5-128 in experimental autoimmune myocarditis and infective endocarditis: comparison with 99mTc-HYNIC-annexin A5. Mol Imaging. doi:10.2310/7290.2014.00049
Maffey KG, Keil LB, DeBari VA (2001) The influence of lipid composition and divalent cations on annexin V binding to phospholipid mixtures. Ann Clin Lab Sci 31(1):85–90
Belhocine TZ, Blankenberg FG, Kartachova MS, Stitt LW, Vanderheyden JL, Hoebers FJ, Van de Wiele C (2015) 99mTc-Annexin A5 quantification of apoptotic tumor response: a systematic review and meta-analysis of clinical imaging trials. Eur J Nucl Med Mol Imaging 42(13):2083–2097. doi:10.1007/s00259-015-3152-0
Boersma HH, Kietselaer BL, Stolk LM, Bennaghmouch A, Hofstra L, Narula J, Heidendal GA, Reutelingsperger CP (2005) Past, present, and future of annexin A5: from protein discovery to clinical applications. J Nucl Med 46(12):2035–2050
Schutters K, Reutelingsperger C (2010) Phosphatidylserine targeting for diagnosis and treatment of human diseases. Apoptosis 15(9):1072–1082. doi:10.1007/s10495-010-0503-y
Alberts B, Johnson A, Lewis J, Raff M, Roberts K, Walter P (2002) The lipid bilayer. In: Molecular biology of the cell, 4th edn. Garland Science, New York. https://www.ncbi.nlm.nih.gov/books/NBK26871/
Hullin-Matsuda F, Makino A, Murate M, Kobayashi T (2016) Probing phosphoethanolamine-containing lipids in membranes with duramycin/cinnamycin and aegerolysin proteins. Biochimie 130:81–90. doi:10.1016/j.biochi.2016.09.020
Post JA, Verkleij AJ, Langer GA (1995) Organization and function of sarcolemmal phospholipids in control and ischemic/reperfused cardiomyocytes. J Mol Cell Cardiol 27(2):749–760
Emoto K, Kobayashi T, Yamaji A, Aizawa H, Yahara I, Inoue K, Umeda M (1996) Redistribution of phosphatidylethanolamine at the cleavage furrow of dividing cells during cytokinesis. Proc Natl Acad Sci USA 93(23):12867–12872
Sprong H, van der Sluijs P, van Meer G (2001) How proteins move lipids and lipids move proteins. Nat Rev Mol Cell Biol 2(7):504–513
van der Wel PC, Pott T, Morein S, Greathouse DV, Koeppe RE 2nd, Killian JA (2000) Tryptophan-anchored transmembrane peptides promote formation of nonlamellar phases in phosphatidylethanolamine model membranes in a mismatch-dependent manner. BioChemistry 39(11):3124–3133
Vance JE, Tasseva G (2013) Formation and function of phosphatidylserine and phosphatidylethanolamine in mammalian cells. Biochim Biophys Acta 1831(3):543–554. doi:10.1016/j.bbalip.2012.08.016
Vance JE (2008) Phosphatidylserine and phosphatidylethanolamine in mammalian cells: two metabolically related aminophospholipids. J Lipid Res 49(7):1377–1387. doi:10.1194/jlr.R700020-JLR200
Jin XH, Okamoto Y, Morishita J, Tsuboi K, Tonai T, Ueda N (2007) Discovery and characterization of a Ca2+-independent phosphatidylethanolamine N-acyltransferase generating the anandamide precursor and its congeners. J Biol Chem 282(6):3614–3623. doi:10.1074/jbc.M606369200
Gao X, van der Veen JN, Vance JE, Thiesen A, Vance DE, Jacobs RL (2015) Lack of phosphatidylethanolamine N-methyltransferase alters hepatic phospholipid composition and induces endoplasmic reticulum stress. Biochim Biophys Acta 1852(12):2689–2699. doi:10.1016/j.bbadis.2015.09.006
Steenbergen R, Nanowski TS, Beigneux A, Kulinski A, Young SG, Vance JE (2005) Disruption of the phosphatidylserine decarboxylase gene in mice causes embryonic lethality and mitochondrial defects. J Biol Chem 280(48):40032–40040. doi:10.1074/jbc.M506510200
Leventis PA, Grinstein S (2010) The distribution and function of phosphatidylserine in cellular membranes. Annu Rev Biophys 39:407–427. doi:10.1146/annurev.biophys.093008.131234
Bratton DL, Fadok VA, Richter DA, Kailey JM, Guthrie LA, Henson PM (1997) Appearance of phosphatidylserine on apoptotic cells requires calcium-mediated nonspecific flip-flop and is enhanced by loss of the aminophospholipid translocase. J Biol Chem 272(42):26159–26165
Verhoven B, Schlegel RA, Williamson P (1995) Mechanisms of phosphatidylserine exposure, a phagocyte recognition signal, on apoptotic T lymphocytes. J Exp Med 182(5):1597–1601
Williamson P, Schlegel RA (2002) Transbilayer phospholipid movement and the clearance of apoptotic cells. Biochim Biophys Acta 1585(2–3):53–63
Aoki Y, Uenaka T, Aoki J, Umeda M, Inoue K (1994) A novel peptide probe for studying the transbilayer movement of phosphatidylethanolamine. J Biochem 116(2):291–297
Suzuki J, Umeda M, Sims PJ, Nagata S (2010) Calcium-dependent phospholipid scrambling by TMEM16F. Nature 468(7325):834–838. doi:10.1038/nature09583
Suzuki J, Denning DP, Imanishi E, Horvitz HR, Nagata S (2013) Xk-related protein 8 and CED-8 promote phosphatidylserine exposure in apoptotic cells. Science 341(6144):403–406. doi:10.1126/science.1236758
Barnett Foster D, Abul-Milh M, Huesca M, Lingwood CA (2000) Enterohemorrhagic Escherichia coli induces apoptosis which augments bacterial binding and phosphatidylethanolamine exposure on the plasma membrane outer leaflet. Infect Immun 68(6):3108–3115
Marconescu A, Thorpe PE (2008) Coincident exposure of phosphatidylethanolamine and anionic phospholipids on the surface of irradiated cells. Biochim Biophys Acta 1778(10):2217–2224. doi:10.1016/j.bbamem.2008.05.006
Fadeel B, Gleiss B, Hogstrand K, Chandra J, Wiedmer T, Sims PJ, Henter JI, Orrenius S, Samali A (1999) Phosphatidylserine exposure during apoptosis is a cell-type-specific event and does not correlate with plasma membrane phospholipid scramblase expression. Biochem Biophys Res Commun 266(2):504–511. doi:10.1006/bbrc.1999.1820
Vallabhapurapu SD, Blanco VM, Sulaiman MK, Vallabhapurapu SL, Chu Z, Franco RS, Qi X (2015) Variation in human cancer cell external phosphatidylserine is regulated by flippase activity and intracellular calcium. Oncotarget 6(33):34375–34388. doi:10.18632/oncotarget.6045
Wesselborg S, Engels IH, Rossmann E, Los M, Schulze-Osthoff K (1999) Anticancer drugs induce caspase-8/FLICE activation and apoptosis in the absence of CD95 receptor/ligand interaction. Blood 93(9):3053–3063
Ferraro-Peyret C, Quemeneur L, Flacher M, Revillard JP, Genestier L (2002) Caspase-independent phosphatidylserine exposure during apoptosis of primary T lymphocytes. J Immunol 169(9):4805–4810
Bezabeh T, Mowat MR, Jarolim L, Greenberg AH, Smith IC (2001) Detection of drug-induced apoptosis and necrosis in human cervical carcinoma cells using 1 H NMR spectroscopy. Cell Death Differ 8(3):219–224. doi:10.1038/sj.cdd.4400802
Hamon Y, Broccardo C, Chambenoit O, Luciani MF, Toti F, Chaslin S, Freyssinet JM, Devaux PF, McNeish J, Marguet D, Chimini G (2000) ABC1 promotes engulfment of apoptotic cells and transbilayer redistribution of phosphatidylserine. Nat Cell Biol 2(7):399–406. doi:10.1038/35017029
Umeda M, Emoto K (1999) Membrane phospholipid dynamics during cytokinesis: regulation of actin filament assembly by redistribution of membrane surface phospholipid. Chem Phys Lipids 101(1):81–91
Ran S, Thorpe PE (2002) Phosphatidylserine is a marker of tumor vasculature and a potential target for cancer imaging and therapy. Int J Radiat Oncol Biol Phys 54(5):1479–1484
Stafford JH, Thorpe PE (2011) Increased exposure of phosphatidylethanolamine on the surface of tumor vascular endothelium. Neoplasia 13(4):299–308
Niu G, Chen X (2010) Apoptosis imaging: beyond annexin V. J Nucl Med 51(11):1659–1662. doi:10.2967/jnumed.110.078584
Guder A, Wiedemann I, Sahl HG (2000) Posttranslationally modified bacteriocins: the lantibiotics. Biopolymers 55(1):62–73. doi:10.1002/1097-0282(2000)55:1<62::AID-BIP60>3.0.CO;2-Y
Kessler H, Steuernagel S, Will M, Jung G, Kellner R, Gillessen D, Kamiyama T (1988) The structure of the polycyclic nonadecapeptide Ro 09-0198. Helv Chim Acta 71(8):1924–1929. doi:10.1002/hlca.19880710811
Zhao M (2011) Lantibiotics as probes for phosphatidylethanolamine. Amino Acids 41(5):1071–1079. doi:10.1007/s00726-009-0386-9
Machaidze G, Ziegler A, Seelig J (2002) Specific binding of Ro 09-0198 (cinnamycin) to phosphatidylethanolamine: a thermodynamic analysis. BioChemistry 41(6):1965–1971
Machaidze G, Seelig J (2003) Specific binding of cinnamycin (Ro 09-0198) to phosphatidylethanolamine. Comparison between micellar and membrane environments. BioChemistry 42(43):12570–12576. doi:10.1021/bi035225b
Iwamoto K, Hayakawa T, Murate M, Makino A, Ito K, Fujisawa T, Kobayashi T (2007) Curvature-dependent recognition of ethanolamine phospholipids by duramycin and cinnamycin. Biophys J 93(5):1608–1619. doi:10.1529/biophysj.106.101584
Choung SY, Kobayashi T, Takemoto K, Ishitsuka H, Inoue K (1988) Interaction of a cyclic peptide, Ro09-0198, with phosphatidylethanolamine in liposomal membranes. Biochim Biophys Acta 940(2):180–187
Cotter PD, Hill C, Ross RP (2005) Bacteriocins: developing innate immunity for food. Nat Rev Microbiol 3(10):777–788. doi:10.1038/nrmicro1273
Makino A, Baba T, Fujimoto K, Iwamoto K, Yano Y, Terada N, Ohno S, Sato SB, Ohta A, Umeda M, Matsuzaki K, Kobayashi T (2003) Cinnamycin (Ro 09-0198) promotes cell binding and toxicity by inducing transbilayer lipid movement. J Biol Chem 278(5):3204–3209. doi:10.1074/jbc.M210347200
Rzeznicka II, Sovago M, Backus EH, Bonn M, Yamada T, Kobayashi T, Kawai M (2010) Duramycin-induced destabilization of a phosphatidylethanolamine monolayer at the air-water interface observed by vibrational sum-frequency generation spectroscopy. Langmuir 26(20):16055–16062. doi:10.1021/la1028965
Wakamatsu K, Choung SY, Kobayashi T, Inoue K, Higashijima T, Miyazawa T (1990) Complex formation of peptide antibiotic Ro09-0198 with lysophosphatidylethanolamine: 1 H NMR analyses in dimethyl sulfoxide solution. BioChemistry 29(1):113–118
Hou S, Johnson SE, Zhao M (2015) A one-step staining probe for phosphatidylethanolamine. ChemBioChem. doi:10.1002/cbic.201500127
Broughton LJ, Giuntini F, Savoie H, Bryden F, Boyle RW, Maraveyas A, Madden LA (2016) Duramycin-porphyrin conjugates for targeting of tumour cells using photodynamic therapy. J Photochem Photobiol B 163:374–384. doi:10.1016/j.jphotobiol.2016.09.001
Li Z, Wells CW, Esmon CT, Zhao M (2009) Phosphatidylethanolamine at the endothelial surface of aortic flow dividers. J Thromb Haemost 7(1):227–229. doi:10.1111/j.1538-7836.2008.03193.x
Zhao M, Li Z (2012) A single-step kit formulation for the 99mTc-labeling of HYNIC-duramycin. Nucl Med Biol 39(7):1006–1011. doi:10.1016/j.nucmedbio.2012.03.006
Wang L, Wang F, Fang W, Johnson SE, Audi S, Zimmer M, Holly TA, Lee DC, Zhu B, Zhu H, Zhao M (2015) The feasibility of imaging myocardial ischemic/reperfusion injury using 99mTc-labeled duramycin in a porcine model. Nucl Med Biol 42(2):198–204. doi:10.1016/j.nucmedbio.2014.09.002
Elvas F, Vangestel C, Rapic S, Verhaeghe J, Gray B, Pak K, Stroobants S, Staelens S, Wyffels L (2015) Characterization of [99mTc]duramycin as a SPECT imaging agent for early assessment of tumor apoptosis. Mol Imaging Biol 17(6):838–847. doi:10.1007/s11307-015-0852-6
Yao S, Hu K, Tang G, Liang X, Du K, Nie D, Jiang S, Zang L (2014) Positron emission tomography imaging of cell death with [(18)F]FPDuramycin. Apoptosis 19(5):841–850. doi:10.1007/s10495-013-0964-x
Jones CJ, Thornback JR (2007) Diagnostic medicine. In: Medicinal applications of coordination chemistry. Royal Society of Chemistry, Cambridge, pp 101–200. doi:10.1039/9781847557759-00101
Hanahan D, Weinberg RA (2011) Hallmarks of cancer: the next generation. Cell 144(5):646–674. doi:10.1016/j.cell.2011.02.013
Wong RS (2011) Apoptosis in cancer: from pathogenesis to treatment. J Exp Clin Cancer Res 30:87. doi:10.1186/1756-9966-30-87
Goodwin RA, Asmis TR (2009) Overview of systemic therapy for colorectal cancer. Clin Colon Rectal Surg 22(4):251–256. doi:10.1055/s-0029-1242465
Erdem ZN, Schwarz S, Drev D, Heinzle C, Reti A, Heffeter P, Hudec X, Holzmann K, Grasl-Kraupp B, Berger W, Grusch M, Marian B (2017) Irinotecan upregulates fibroblast growth factor receptor 3 expression in colorectal cancer cells, which mitigates irinotecan-induced apoptosis. Trans Int Soc Cell 10(3):332–339. doi:10.1016/j.tranon.2017.02.004
Elvas F, Vangestel C, Pak K, Vermeulen P, Gray B, Stroobants S, Staelens S, Wyffels L (2016) Early prediction of tumor response to treatment: preclinical validation of 99mTc-duramycin. J Nucl Med 57(5):805–811. doi:10.2967/jnumed.115.168344
Luo R, Niu L, Qiu F, Fang W, Fu T, Zhao M, Zhang YJ, Hua ZC, Li XF, Wang F (2016) Monitoring apoptosis of breast cancer xenograft after paclitaxel treatment with 99mTc-labeled duramycin SPECT/CT. Mol Imaging. doi:10.1177/1536012115624918
Rahmanian N, Hosseinimehr SJ, Khalaj A (2016) The paradox role of caspase cascade in ionizing radiation therapy. J Biomed Sci 23(1):88. doi:10.1186/s12929-016-0306-8
De Saint-Hubert M, Bauwens M, Mottaghy FM (2014) Molecular imaging of apoptosis for early prediction of therapy efficiency. Curr Pharm Des 20(14):2319–2328
Van de Wiele C, Vermeersch H, Loose D, Signore A, Mertens N, Dierckx R (2004) Radiolabeled annexin-V for monitoring treatment response in oncology. Cancer Biother Radiopharm 19(2):189–194. doi:10.1089/108497804323071968
Labi V, Erlacher M (2015) How cell death shapes cancer. Cell Death Dis 6:e1675. doi:10.1038/cddis.2015.20
Elvas F, Boddaert J, Vangestel C, Pak K, Gray B, Kumar-Singh S, Staelens S, Stroobants S, Wyffels L (2016) 99mTc-Duramycin SPECT imaging of early tumor response to targeted therapy: a comparison with 18F-FDG PET. J Nucl Med. doi:10.2967/jnumed.116.182014
Kostakoglu L, Goldsmith SJ (2003) 18F-FDG PET evaluation of the response to therapy for lymphoma and for breast, lung, and colorectal carcinoma. J Nucl Med 44(2):224–239
Ben-Haim S, Ell P (2009) 18F-FDG PET and PET/CT in the evaluation of cancer treatment response. J Nucl Med 50(1):88–99. doi:10.2967/jnumed.108.054205
Chang JM, Lee HJ, Goo JM, Lee HY, Lee JJ, Chung JK, Im JG (2006) False positive and false negative FDG-PET scans in various thoracic diseases. Korean J Radiol 7(1):57–69. doi:10.3348/kjr.2006.7.1.57
Ran S, Downes A, Thorpe PE (2002) Increased exposure of anionic phospholipids on the surface of tumor blood vessels. Cancer Res 62(21):6132–6140
Galluzzi L, Vitale I, Vacchelli E, Kroemer G (2011) Cell death signaling and anticancer therapy. Front Oncol 1:1–18. doi:10.3389/fonc.2011.00005
Konstantinidis K, Whelan RS, Kitsis RN (2012) Mechanisms of cell death in heart disease. Arterioscler Thromb Vasc Biol 32(7):1552–1562. doi:10.1161/ATVBAHA.111.224915
Narula J, Zaret BL (2002) Noninvasive detection of cell death: from tracking epitaphs to counting coffins. J Nucl Cardiol 9(5):554–560
Musters RJ, Otten E, Biegelmann E, Bijvelt J, Keijzer JJ, Post JA, Op den Kamp JA, Verkleij AJ (1993) Loss of asymmetric distribution of sarcolemmal phosphatidylethanolamine during simulated ischemia in the isolated neonatal rat cardiomyocyte. Circ Res 73(3):514–523
Post JA, Clague JR, Langer GA (1993) Sarcolemmal phospholipid asymmetry and Ca fluxes on metabolic inhibition of neonatal rat heart cells. Am J Physiol 265(2 Pt 2):H461–H468
Salerno M, Beller GA (2009) Noninvasive assessment of myocardial perfusion. Circulation 2(5):412–424. doi:10.1161/circimaging.109.854893
Audi S, Li Z, Capacete J, Liu Y, Fang W, Shu LG, Zhao M (2012) Understanding the in vivo uptake kinetics of a phosphatidylethanolamine-binding agent 99mTc-duramycin. Nucl Med Biol 39(6):821–825. doi:10.1016/j.nucmedbio.2012.02.004
Taki J, Higuchi T, Kawashima A, Tait JF, Kinuya S, Muramori A, Matsunari I, Nakajima K, Tonami N, Strauss HW (2004) Detection of cardiomyocyte death in a rat model of ischemia and reperfusion using 99mTc-labeled annexin V. J Nucl Med 45(9):1536–1541
Dumont EA, Hofstra L, van Heerde WL, van den Eijnde S, Doevendans PA, DeMuinck E, Daemen MA, Smits JF, Frederik P, Wellens HJ, Daemen MJ, Reutelingsperger CP (2000) Cardiomyocyte death induced by myocardial ischemia and reperfusion: measurement with recombinant human annexin-V in a mouse model. Circulation 102(13):1564–1568
Kajstura J, Cheng W, Reiss K, Clark WA, Sonnenblick EH, Krajewski S, Reed JC, Olivetti G, Anversa P (1996) Apoptotic and necrotic myocyte cell deaths are independent contributing variables of infarct size in rats. Lab Invest 74(1):86–107
Kostin S, Pool L, Elsasser A, Hein S, Drexler HC, Arnon E, Hayakawa Y, Zimmermann R, Bauer E, Klovekorn WP, Schaper J (2003) Myocytes die by multiple mechanisms in failing human hearts. Circ Res 92(7):715–724. doi:10.1161/01.RES.0000067471.95890.5C
Tabas I, Seimon T, Timmins J, Li G, Lim W (2009) Macrophage apoptosis in advanced atherosclerosis. Ann N Y Acad Sci 1173(Suppl 1):E40–E45. doi:10.1111/j.1749-6632.2009.04957.x
Andres V, Pello OM, Silvestre-Roig C (2012) Macrophage proliferation and apoptosis in atherosclerosis. Curr Opin Lipidol 23(5):429–438. doi:10.1097/MOL.0b013e328357a379
Liu Z, Larsen BT, Lerman LO, Gray BD, Barber C, Hedayat AF, Zhao M, Furenlid LR, Pak KY, Woolfenden JM (2016) Detection of atherosclerotic plaques in ApoE-deficient mice using 99mTc-duramycin. Nucl Med Biol 43(8):496–505. doi:10.1016/j.nucmedbio.2016.05.007
Broughton BR, Reutens DC, Sobey CG (2009) Apoptotic mechanisms after cerebral ischemia. Stroke 40(5):e331–e339. doi:10.1161/STROKEAHA.108.531632
Zhang Y, Stevenson GD, Barber C, Furenlid LR, Barrett HH, Woolfenden JM, Zhao M, Liu Z (2013) Imaging of rat cerebral ischemia-reperfusion injury using 99mTc-labeled duramycin. Nucl Med Biol 40(1):80–88. doi:10.1016/j.nucmedbio.2012.09.004
Audi SH, Jacobs ER, Zhao M, Roerig DL, Haworth ST, Clough AV (2015) In vivo detection of hyperoxia-induced pulmonary endothelial cell death using 99mTc-duramycin. Nucl Med Biol 42(1):46–52. doi:10.1016/j.nucmedbio.2014.08.010
Clough AV, Audi SH, Haworth ST, Roerig DL (2012) Differential lung uptake of 99mTc-hexamethylpropyleneamine oxime and 99mTc-duramycin in the chronic hyperoxia rat model. J Nucl Med 53(12):1984–1991. doi:10.2967/jnumed.112.108498
Johnson SE, Li Z, Liu Y, Moulder JE, Zhao M (2013) Whole-body imaging of high-dose ionizing irradiation-induced tissue injuries using 99mTc-duramycin. J Nucl Med 54(8):1397–1403. doi:10.2967/jnumed.112.112490
Medhora M, Haworth S, Liu Y, Narayanan J, Gao F, Zhao M, Audi S, Jacobs ER, Fish BL, Clough AV (2016) Biomarkers for radiation pneumonitis using noninvasive molecular imaging. J Nucl Med 57(8):1296–1301. doi:10.2967/jnumed.115.160291
Gao F, Fish BL, Moulder JE, Jacobs ER, Medhora M (2013) Enalapril mitigates radiation-induced pneumonitis and pulmonary fibrosis if started 35 days after whole-thorax irradiation. Radiat Res 180(5):546–552. doi:10.1667/RR13350.1
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Elvas, F., Stroobants, S. & Wyffels, L. Phosphatidylethanolamine targeting for cell death imaging in early treatment response evaluation and disease diagnosis. Apoptosis 22, 971–987 (2017). https://doi.org/10.1007/s10495-017-1384-0
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10495-017-1384-0