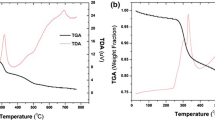
Abstract
Monolithic cesium ion (Cs+) adsorbents were synthesized via the directional freezing of a silica hydrogel containing ammonium molybdophosphate (AMP) particles, followed by freeze-drying. The adsorbents have a honeycomb-like structure with nearly straight microchannels (approximately 21 µm in diameter) running through them and with AMP particles partially embedded intact within the channel walls. Because of its honeycomb-like structure, the adsorbent, denoted as AMP silica microhoneycomb (AMP-SMH), achieves a significantly lower pressure drop than a typical column packed with spherical particles with similar diffusion path lengths for Cs+ when water was passed through it (about 35-times lower). Comparison of breakthrough curves between the AMP-SMH and columns packed with particles by numerical simulation also indicates that AMP-SMH shows shorter length of unused bed values. These results demonstrate that the AMP-SMH shows a high performance in the continuous separation of Cs+ due to their unique microhoneycomb structure.












Similar content being viewed by others
References
Abusafa, A., Yücel, H.: Removal of 137Cs from aqueous solutions using different cationic forms of a natural zeolite clinoptilolite. Sep. Purif. Technol. 28(2), 103–116 (2002)
Anthony, R.G., Dosch, R.G., Gu, D., Philip, C.V.: Use of silicotitanates for removing cesium and strontium from defense waste. Ind. Eng. Chem. Res. 33(11), 2702–2705 (1994)
Audi, G., Bersillon, O., Blachot, J., Wapstra, A.H.: The NUBASE evaluation of nuclear and decay properties. Nucl. Phys. A 729(1), 3–128 (2003)
Bird, R.B., Stewart, W.E., Lightfoot, E.N.: Transport Phenomena, 2nd edn. Wiley, New York (2002)
Bridgeman, A.J.: Density functional study of the vibrational frequencies of α-Keggin heteropolyanions. Chem. Phys. 287(1–2), 55–69 (2003)
Doležal, J., Stejskal, J., Tympl, M., Kouřím, V.: Improved inorganic ion-exchangers. J. Radioanal. Nucl. Chem. 21(2), 381–387 (1974)
Dozol, J.F., Dozol, M., Macias, R.M.: Extraction of strontium and cesium by dicarbollides, crown ethers and functionalized calixarenes. J. Incl. Phenom. Macrocycl. Chem. 38(1–4), 1–22 (2000)
Endo, Y., Wu, Y., Mimura, H., Niibori, Y., Ozawa, M.: Selective uptake of cesium ions on AMP-loaded silica gels. J. Ion. Exch. 18(4), 444–449 (2007)
Henley, E.J., Seader, J.D., Roper, D.K.: Separation Process Principles, 3rd edn. Wiley, New York (2011)
Kozeny, J.: Ueber kapillare leitung des wassers im boden. Sitzungsber. Akad. Wiss. Wien. 136, 271–306 (1927)
Lin, Y., Fryxell, G.E., Wu, H., Engelhard, M.: Selective sorption of cesium using self-assembled monolayers on mesoporous supports. Environ. Sci. Technol. 35(19), 3962–3966 (2001)
Mimura, H., Kanno, T.: Distribution and fixation of cesium and strontium in zeolite A and chabazite. J. Nucl. Sci. Technol. 22(4), 284–291 (1985)
Mimura, H., Saito, M., Akiba, K., Onodera, Y.: Selective uptake of cesium by ammonium molybdophosphate (AMP)-calcium alginate composites. J. Nucl. Sci. Technol. 38(10), 872–878 (2001)
Mukai, S.R., Nishihara, H., Tamon, H.: Formation of monolithic silica gel microhoneycombs (SMHs) using pseudosteady state growth of microstructural ice crystals. Chem. Commun. (7), 874–875 (2004). https://doi.org/10.1039/B316597C
Nilchi, A., Saberi, R., Moradi, M., Azizpour, H., Zarghami, R.: Adsorption of cesium on copper hexacyanoferrate–PAN composite ion exchanger from aqueous solution. Chem. Eng. J. 172(1), 572–580 (2011)
Nishihara, H., Mukai, S.R., Yamashita, D., Tamon, H.: Ordered macroporous silica by ice templating. Chem. Mater. 17(3), 683–689 (2005)
Parajuli, D., Takahashi, A., Tanaka, H., Sato, M., Fukuda, S., Kamimura, R., Kawamoto, T.: Variation in available cesium concentration with parameters during temperature induced extraction of cesium from soil. J. Environ. Radioact. 140, 78–83 (2015)
Park, Y., Lee, Y.-C., Shin, W.S., Choi, S.-J.: Removal of cobalt, strontium and cesium from radioactive laundry wastewater by ammonium molybdophosphate–polyacrylonitrile (AMP–PAN). Chem. Eng. J. 162(2), 685–695 (2010)
Popa, A., Sasca, V., Holclajtner-Antunović, I.: The influence of surface coverage on textural, structural and catalytic properties of cesium salts of 12-molybdophosphoric acid supported on SBA-15 mesoporous silica. Microporous Mesporous Mater. 156, 127–137 (2012)
Prout, W.E., Russell, E.R., Groh, H.J.: Ion exchange absorption of cesium by potassium hexacyanocobalt (II) ferrate (II). J. Inorg. Nucl. Chem. 27(2), 473–479 (1965)
Rao, K.L.N., Shukla, J.P., Venkataramani, B.: Electron irradiation studies on ammonium molybdophosphate. J. Radioanal. Nucl. Chem. 189(1), 107–114 (1995)
Ruthven, D.M.: Principles of Adsorption and Adsorption Processes. Wiley, New York (1984)
Samanta, S.K., Theyyunni, T.K., Misra, B.M.: Column behaviour of resorcinol-formaldehyde polycondensate resin for radiocesium removal from simulated radwaste solution. J. Nucl. Sci. Technol. 32(5), 425–429 (1995)
Sangvanich, T., Sukwarotwat, V., Wiacek, R.J., Grudzien, R.M., Fryxell, G.E., Addleman, R.S., Timchalk, C., Yantasee, W.: Selective capture of cesium and thallium from natural waters and simulated wastes with copper ferrocyanide functionalized mesoporous silica. J. Hazard. Mater. 182(1–3), 225–231 (2010)
Satyanarayana, J., Murthy, G.S., Sasidhar, P.: Adsorption studies of cesium on a new inorganic exchanger ammonium molybdophosphate-alumina (AMP-Al2O3). J. Radioanal. Nucl. Chem. 242(1), 11–16 (1999)
Sing, K.S.W.: Reporting physisorption data for gas/solid systems with special reference to the determination of surface area and porosity (recommendations 1984). Pure Appl. Chem. 57(4), 603–619 (1985)
Smit, J.V.R.: Ammonium salts of the heteropolyacids as cation exchangers. Nature 181(4622), 1530–1531 (1958)
Sydorchuk, V., Khalameida, S., Skubiszewska-Zięba, J., Leboda, R.: Synthesis and structure of AMP/oxide support. J. Therm. Anal. Calorim. 103(1), 257–265 (2011)
Todd, T.A., Mann, N.R., Tranter, T.J., Šebesta, F., John, J., Motl, A.: Cesium sorption from concentrated acidic tank wastes using ammonium molybdophosphate-polyacrylonitrile composite sorbents. J. Radioanal. Nucl. Chem. 254(1), 47–52 (2002)
Tranter, T.J., Herbst, R.S., Todd, T.A., Olson, A.L., Eldredge, H.B.: Evaluation of ammonium molybdophosphate-polyacrylonitrile (AMP-PAN) as a cesium selective sorbent for the removal of 137Cs from acidic nuclear waste solutions. Adv. Environ. Res. 6(2), 107–121 (2002)
Yasunari, T.J., Stohl, A., Hayano, R.S., Burkhart, J.F., Eckhardt, S., Yasunari, T.: Cesium-137 deposition and contamination of Japanese soils due to the Fukushima nuclear accident. Proc. Natl. Acad. Sci. USA 108(49), 19530–19534: (2011)
Yasutaka, T., Kawamoto, T., Komai, T.: Applicability of the acid extraction method to radioactive caesium contaminated soil. Radioisotopes 62(4), 211–218 (2013)
Yoshida, S., Kimura, Y., Ogino, I., Mukai, S.R.: Synthesis of a microhoneycomb-type silica-supported ammonium molybdophosphate for cesium separation. J. Chem. Eng. Jpn. 46(9), 616–619 (2013)
Acknowledgements
This work was supported by the Japan Society for the promotion of Science (JSPS) Grant-in-Aid for Scientific Research (B) 24360324.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Yoshida, S., Iwamura, S., Ogino, I. et al. Continuous-flow separation of cesium ion by ammonium molybdophosphate immobilized in a silica microhoneycomb (AMP-SMH). Adsorption 25, 1089–1098 (2019). https://doi.org/10.1007/s10450-019-00060-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10450-019-00060-2