Abstract
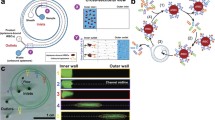
This study describes a non-dilutive high-gradient magnetic separation (HGMS) device intended to continuously remove malaria-infected red blood cells (iRBCs) from the circulation. A mesoscale prototype device with disposable photo-etched ferromagnetic grid and reusable permanent magnet was designed with a computationally-optimized magnetic force. The prototype device was evaluated in vitro using a non-pathogenic analog for malaria-infected blood, comprised of 24% healthy RBCs, 6% human methemoglobin RBCs (metRBCs), and 70% phosphate buffer solution (PBS). The device provided a 27.0 ± 2.2% reduction of metRBCs in a single pass at a flow rate of 77 μL min−1. This represents a clearance rate over 380 times greater throughput than microfluidic devices reported previously. These positive results encourage development of a clinical scale system that would economize time and donor blood for treating severe malaria.







Similar content being viewed by others
References
Ahn, S. Y., M. Y. Shin, Y. A. Kim, J. A. Yoo, D. H. Kwak, Y. J. Jung, G. Jun, S. H. Ryu, J. S. Yeom, J. Y. Ahn, J. Y. Chai, and J. W. Park. Magnetic separation: a highly effective method for synchronization of cultured erythrocytic Plasmodium falciparum. Parasitol. Res. 102:1195–1200, 2008.
Bhakdi, S. C., A. Ottinger, S. Somsri, P. Sratongno, P. Pannadaporn, P. Chimma, P. Malasit, K. Pattanapanyasat, and H. P. H. Neumann. Optimized high gradient magnetic separation for isolation of Plasmodium-infected red blood cells. Malar. J. 9:38, 2010.
Boctor, F. N. Red blood cell exchange transfusion as an adjunct treatment for severe pediatric falciparum malaria, using automated or manual procedures. Pediatrics 116:e592–e595, 2005.
Chikov, V., A. Kuznetsov, and A. Shapiro. Single cell magnetophoresis and its diagnostic value. J. Magn. Magn. Mater. 122(122):367–370, 1993.
Chotivanich, K., R. Udomsangpetch, J. A. Simpson, P. Newton, S. Pukrittayakamee, S. Looareesuwan, and N. J. White. Parasite multiplication potential and the severity of falciparum malaria. J. Infect. Dis. 181:1206–1209, 2000.
Cranston, H., C. Boylan, G. Carroll, S. Sutera, J. Williamson, I. Gluzman, and D. Krogstad. Plasmodium falciparum maturation abolishes physiologic red cell deformability. Science 223:400–403, 1984.
Deshpande, A., S. Kalgutkar, and S. Udani. Red cell exchange using cell separator (therapeutic erythrocytapheresis) in two children with acute severe malaria. J Assoc Physicians India 51:925–926, 2003.
Furdui, V. I., and D. J. Harrison. Immunomagnetic T cell capture from blood for PCR analysis using microfluidic systems. Lab Chip 4:614–618, 2004.
Graham, M. D. Efficiency comparison of two preparative mechanisms for magnetic separation of erythrocytes from whole blood. J. Appl. Phys. 52:2578–2580, 1981.
Hackett, S., J. Hamzah, T. M. E. Davis, and T. G. St Pierre. Magnetic susceptibility of iron in malaria-infected red blood cells. Biochim. Biophys. Acta Mol. Basis Dis 1792:93–99, 2009.
Hall, A. Exchange transfusion and quinine concentrations in falciparum malaria. Br. Med. J. 291:1169–1170, 1985.
Han, K.-H., and A. B. Frazier. Paramagnetic capture mode magnetophoretic microseparator for high efficiency blood cell separations. Lab Chip 6:265–273, 2006.
Iliescu, C., G. Xu, E. Barbarini, M. Avram, and A. Avram. Microfluidic device for continuous magnetophoretic separation of white blood cells. Microsyst. Technol. 15:1157–1162, 2009.
Kang, J. H., S. Krause, H. Tobin, A. Mammoto, M. Kanapathipillai, and D. E. Ingber. A combined micromagnetic-microfluidic device for rapid capture and culture of rare circulating tumor cells. Lab Chip 12:2175, 2012.
Karl, S., M. David, L. Moore, B. T. Grimberg, P. Michon, I. Mueller, M. Zborowski, and P. A. Zimmerman. Enhanced detection of gametocytes by magnetic deposition microscopy predicts higher potential for Plasmodium falciparum transmission. Malar. J. 7:66, 2008.
Kong, T. F., W. Ye, W. K. Peng, H. W. Hou, P. R. Preiser, N.-T. Nguyen, and J. Han. Enhancing malaria diagnosis through microfluidic cell enrichment and magnetic resonance relaxometry detection. Nat. Sci. Rep. 5:1–12, 2015.
Kramer, S. L., C. C. Campbell, and R. E. Moncrieff. Fulminant Plasmodium falciparum infection treated with exchange blood transfusion. J. Am. Med. Assoc. 249:244–245, 1983.
Macallan, D. C., M. Pocock, E. Bishop, D. H. Bevan, J. Parker-Williams, T. Harrison, and G. T. Robinson. Automated erythrocytapheresis in the treatment of severe falciparum malaria. J. Infect. 39:233–236, 1999.
Martin, A. B., and J. F. Antaki. Comparison of clearance of malaria-infected erythrocytes by exchange transfusion and high gradient magnetic apheresis. 2017
Melville, D., F. Paul, and S. Roath. Direct magnetic separation of red cells from whole blood. Nature 255:706, 1975.
Nalbandian, R. M., D. W. Sammons, M. Manley, L. Xie, C. R. Sterling, N. B. Egen, and B. A. Gingras. A molecular-based magnet test for malaria. Am. J. Clin. Pathol. 103:57–64, 1995.
Nam, J., H. Huang, H. Lim, C. Lim, and S. Shin. Magnetic separation of malaria-infected red blood cells in various developmental stages. Anal. Chem. 85:7316–7323, 2013.
Oka, T., H. Kanayama, S. Fukui, J. Ogawa, T. Sato, M. Ooizumi, T. Terasawa, Y. Itoh, and R. Yabuno. Application of HTS bulk magnet system to the magnetic separation techniques for water purification. Phys. C Supercond 468:2128–2132, 2008.
Owen, C. S. High gradient magnetic separation of erythrocytes. Biophys. J. 22:171–178, 1978.
Pamme, N., and C. Wilhelm. Continuous sorting of magnetic cells via on-chip free-flow magnetophoresis. Lab Chip 6:974, 2006.
Paul, F., S. Roath, D. Melville, D. C. Warhurst, and J. O. S. Osisanya. Separation of malaria-infected erythrocytes from whole blood: use of a selective high-gradient magnetic separation technique. Lancet 318:70–71, 1981.
Phillips, P., S. Nantel, and W. Benny. Exchange transfusion as an adjunct to the treatment of severe falciparum malaria. Rev. Infect. Dis. 12:1100–1108, 1990.
Qu, B. Y., Z. Y. Wu, F. Fang, Z. M. Bai, D. Z. Yang, and S. K. Xu. A glass microfluidic chip for continuous blood cell sorting by a magnetic gradient without labeling. Anal. Bioanal. Chem. 392:1317–1324, 2008.
Ribaut, C., A. Berry, S. Chevalley, K. Reybier, I. Morlais, D. Parzy, F. Nepveu, F. Benoit-Vical, and A. Valentin. Concentration and purification by magnetic separation of the erythrocytic stages of all human Plasmodium species. Malar. J. 7:45, 2008.
Sherman, I. W. Amino acid metabolism and protein synthesis in malarial parasites. Bull. World Health Organ. 55:265–276, 1977.
Takayasu, M., N. Duske, S. R. Ash, and F. J. Friedlaender. HGMS studies of blood cell behavior in plasma. IEEE Trans. Magn. 18:1520–1522, 1982.
Trang, D. T. X., N. T. Huy, T. Kariu, K. Tajima, and K. Kamei. One-step concentration of malarial parasite-infected red blood cells and removal of contaminating white blood cells. Malar. J. 3:7, 2004.
White, N. The parasite clearance curve. Malar. J. 10:278, 2011.
World Health Organization. Guidelines for the Treatment of Malaria. Geneva: WHO Press, 2006.
World Malaria Report. 2015
Wu, W.-T., A. B. Martin, A. Gandini, N. Aubry, M. Massoudi, and J. F. Antaki. Design of microfluidic channels for magnetic separation of malaria-infected red blood cells. Microfluid. Nanofluid. 20:1–11, 2016.
Xia, N., T. P. Hunt, B. T. Mayers, E. Alsberg, G. M. Whitesides, R. M. Westervelt, and D. E. Ingber. Combined microfluidic-micromagnetic separation of living cells in continuous flow. Biomed. Microdevices 8:299–308, 2006.
Zavodnik, I. B., E. A. Lapshina, K. Rekawiecka, L. B. Zavodnik, G. Bartosz, and M. Bryszewska. Membrane effects of nitrite-induced oxidation of human red blood cells. Biochim. Biophys. Acta 1421:306–316, 1999.
Zborowski, M., G. R. Ostera, L. R. Moore, S. Milliron, J. J. Chalmers, and A. N. Schechter. Red blood cell magnetophoresis. Biophys. J. 84:2638–2645, 2003.
Zhang, Y., L. Telleria, J. M. Vinetz, D. Yawn, S. Rossmann, and A. J. Indrikovs. Erythrocytapheresis for Plasmodium falciparum infection complicated by cerebral malaria and hyperparasitemia. J. Clin. Apher. 16:15–18, 2001.
Acknowledgments
Funding was provided by National Institutes of Health (Grant No R01 HL089456).
Conflict of interest
No benefits in any form have been or will be received from a commercial party related directly or indirectly to the subject of this manuscript.
Author information
Authors and Affiliations
Corresponding author
Additional information
Associate Editor Umberto Morbiducci oversaw the review of this article.
Rights and permissions
About this article
Cite this article
Blue Martin, A., Wu, WT., Kameneva, M.V. et al. Development of a High-Throughput Magnetic Separation Device for Malaria-Infected Erythrocytes. Ann Biomed Eng 45, 2888–2898 (2017). https://doi.org/10.1007/s10439-017-1925-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10439-017-1925-2