Abstract
About 96% of all malaria deaths occur in Africa, and the malignant falciparum malaria also originated on the continent. Although falciparum malaria only appeared in the Holocene period, it can be hypothesized that the transfer of malaria parasites from other primates to humans occurred several times in history parallel to human evolution. This study develops the model that examines the possible coexistence of the potential original host apes, human ancestors, and the diverse anopheline mosquito species; and how, where, and when the host switch of these parasites from great apes to humans occurred. Based on the Pliocene-early Pleistocene archaeological sites, it was found that certain early hominin populations could have lived in malaria areas where the anopheline mosquito fauna was moderately diverse. The people of the Lupemban Culture, as well as the Greenlandian and Northgrippian human populations of East and West-Central Africa, lived close to the high diversity of anopheline fauna and the territories of such great apes as Gorilla gorrilla. African mid-Holocene cultures likely came in contact with gorilla populations — the original hosts of Plasmodium falciparum — along the coasts of the Gulf of Guinea and the East African Rift Valley during their migration to southern Africa. The host switch of the ancestor of the falciparum malaria parasite likely occurred in these regions.
Résumé
Environ 96% de tous les décès dus au paludisme surviennent en Afrique, et le paludisme malin à falciparum est également originaire du continent. Bien que le paludisme à falciparum ne soit apparu qu’à l’Holocène, on peut émettre l’hypothèse que le transfert de parasites du paludisme d’autres primates à l’homme s’est produit plusieurs fois dans l’histoire parallèlement à l’évolution humaine. Cette étude développe le modèle qui examine la coexistence possible des singes hôtes d’origine potentiels, des ancêtres humains et des diverses espèces de moustiques anophèles; et comment, où et quand s’est produit le changement d’hôte de ces parasites des grands singes aux humains. Sur la base des sites archéologiques du Pliocène et du Pléistocène inférieur, il a été constaté que certaines populations d’hominidés précoces auraient pu vivre dans des zones de paludisme où la faune de moustiques anophèles était modérément diversifiée. Les peuples de la culture lupemban, ainsi que les populations humaines groenlandaises et nordgrippiennes d’Afrique orientale et centrale occidentale, vivaient à proximité de la grande diversité de la faune anophèle et des territoires de grands singes tels que Gorilla gorrilla. Les cultures africaines du milieu de l’Holocène sont probablement entrées en contact avec des populations de gorilles—les hôtes originaux de Plasmodium falciparum—le long des côtes du golfe de Guinée et de la vallée du Rift est-africain lors de leur migration vers l’Afrique australe. Le changement d’hôte de l’ancêtre du parasite du paludisme falciparum s’est probablement produit dans ces régions.
Similar content being viewed by others
Introduction
Based on the WHO’s World Malaria Report (WHO, 2020), the number of global estimated malaria cases in 2019 was about 229 million worldwide. Of the 87 countries where malaria is endemic, 29 countries accounted for 96% of malaria cases globally. The African region accounted for 215 million cases, and 384,000 deaths were estimated in 2019. In Southeast Asia, 6.3 million malaria cases and 9,000 deaths were recorded in the same year. Five Plasmodium species are known to infect humans, and from these species, Plasmodium vivax (Grassi & Feletti, 1890) and Plasmodium falciparum (Welch, 1897) are responsible for more than 95% of human infections (Larson, 2019). However, the global proportion of cases due to P. vivax was only about 3% in 2019 (Daron et al., 2020). Plasmodium falciparum is responsible for almost all human malaria cases in sub-Saharan Africa.
Malaria in sub-Saharan Africa has been almost exclusively attributed to P. falciparum for a long time, but P. vivax is being reported more frequently from Africa in recent publications. It can be much more prevalent there than previously thought because, in 2015, the direct transmission of the pathogen was already found in 21 of the 47 malaria-endemic countries (Howes et al., 2015). For now, there is evidence of P. vivax in all regions of Africa (Twohig et al., 2019), and it occurs in all areas where the annual precipitation sum is no less than 200 mm. Today, P. falciparum has a worldwide distribution. It is found in the African mainland, Madagascar, the Arabian Peninsula, Latin America, and South Asia (Kigen, 2019).
In contrast to its present-day global occurrence, it is plausible that P. falciparum originated in Africa from a Laverania ancestor due to a host switch between Gorilla species and humans in the early to mid-Holocene era (Loy et al., 2017). Loy et al. (2017) found that the observed level of genetic diversity in P. falciparum could have readily accumulated within the past 10 ky. It could explain why P. falciparum differs from other human malaria Plasmodium species in many aspects of its biology, particularly its virulence (Sundararaman et al., 2016). However, this very late origin time can be only valid for the appearance of the present-day widespread and highly malignant evolutionary line of P. falciparum. Silva et al. (2011) found that the divergence of P. falciparum and Plasmodium reichenowi Sluiter, Swellengrebel, and Ihle, 1922 (a chimpanzee malaria parasite) could have occurred ca. 3.0–5.5 Mya. Interestingly, the average divergence time value, 4.25 Mya, is close to the 4.2–3.8 Mya-age fossils of the earliest-known hominin, Australopithecus anamensis (Ward et al., 1999). This early australopithecine clearly shows the anatomical traits of the human clade but does not exhibit anatomical features characteristic of the chimpanzee lineage (Kimbel et al., 2006).
Although indirect evidence suggests that different malaria parasites influenced the human evolution both in Africa and Europe (Trájer, 2021; Trájer et al., 2020), the estimation of the possible time and place of the host switch of ancient Plasmodium species — apart from the molecular genetic evidence — is difficult to test. There are no elaborated and widely accepted methods that could help to test the genetic-based hypothesis. The direct evidence for the genetic traits of the parasites and other infectious agents is rare. The exceptions include the leishmaniasis-infected ancient Egyptian mummies and the remains of formerly malaria-infected ancient Roman individuals (Marciniak et al., 2016; Zink et al., 2006). Due to the relative rarity of well-preserved human remains older than the Holocene era and the effect of the diagenetic processes during fossilization, the probability of finding the molecular trait of ancient parasites is low. However, the extraction of nuclear sequences from the 430,000 years old Sima de Los Huesos hominin remains signals more possibilities in the near future (Meyer et al., 2016).
The presence of falciparum malaria and tuberculosis were also confirmed in ancient Egyptian mummies (Lalremruata et al., 2013). Sometimes, as indirect evidence, palaeopathological signs like the leishmaniasis-related bone lesions or cribra orbitalia in the case of malaria infections indicate the past presence of a parasitic disease in an area (e.g., Costa et al., 2009; Gerszten et al., 2012; Gowland & Western, 2012; Obertová & Thurzo, 2008). It is known that exostosis was a prevalent disease among Homo erectus individuals, and the clear skeletal signs of arthritis, perhaps the tuberculosis of the spine, and generalized syphilitic osteomyelitis-like signs have been observed in Neanderthals (Theodorakopoulou & Karamanou, 2020). However, one must exercise caution in interpreting the ancient osteologic malformations. On the one hand, ancient infectious diseases are not known, and a given skeletal lesion can be traced back to different causes. On the other hand, infections, tumors, malnutrition, and autoimmune processes can also result in similar skeletal lesions. Of greater relevance to the present study is that the earliest hyperostotic lesion was observed on a 1.5-million-year-old hominin remain found in the Olduvai Gorge, Tanzania (Domínguez-Rodrigo et al., 2012). Although porotic hyperostosis can also be the consequence of other hemolytic anemias, like sickle cell and thalassemia, the strongest correlation exists between malaria and the prevalence of this lesion in the tropical regions (Angel, 1966). It should also not be forgotten that high frequencies of α-thalassemia and sickle cell anemia result from natural selection caused by malaria (Flint et al., 1986; Kariuki & Williams, 2020; Luzzatto, 2012).
A possible indirect way to test hypotheses related to the past presence of malaria in an area, via a coexistence approach, is to investigate whether the elements of the vector chain, namely the anopheline mosquito vectors, the possible host primates, and human hosts, could be present in an area at the same time or not (Trájer, 2021). It can be hypothesized that the diversity of the anopheline malaria vectors influences the possibility of host switch due to several causes. For example, human-made fragmented habitats can increase the anopheline mosquito diversity, leading to the higher prevalence of both human and simian malaria in these sites (Multini et al., 2020). It is also known that the areas with the greatest Anopheles abundance are also those where the highest diversity of anopheline mosquito species can be observed (Gomes et al., 2020). These facts indicate a strong correlation between anopheline mosquito diversity, abundance, and malaria prevalence. Given these relationships, it would be difficult to assume that the likelihood of a host change of malaria parasites would not be substantially affected by the same factors. It was observed that even in the case of two malaria mosquito species, the genetic diversity of P. falciparum could be high (Annan et al., 2007), and this genotypic diversity is an essential factor in the host switch probability. Haemosporidians (Phylum Apicomplexa), including malaria parasitic agents, are transmitted by several hematophagous vectors like mosquitoes, sandflies, louse flies, biting midges, and blackflies. The mosaic of the competent vectors and the different parasites related to the susceptible reptile, bird, and mammal species indicates that vector switches between the vector insects occurred multiple times (Martinsen et al., 2008). It is very plausible that the ability of malaria parasites to radiate in the world was strongly influenced by their propensity to shift among different host species, as observed in the Amazonian avian malaria species (Fecchio et al., 2018).
Host switches occurred between primates and ancient humans. It is known that primates have several of their own Plasmodium pathogens. For example, Pan and Gorilla species harbor several protozoan species in close relationship with human P. vivax (Loy et al., 2018). Japanese macaques and cynomolgus macaques also have closely related P. vivax-like malaria parasites (Tachibana et al., 2015). All these show that primates are hosts of several malaria parasites that, based on the evolutionary process of host switch, could become the agents of human malaria forms. However, considering the possibility of host switch, it should be known that neither the malaria vectors’ human blood preference nor the parasite transmission potential is the same. For example, the potential malaria vectors in Hungary (Central Europe) do not prefer human blood (Trájer, 2018), which is intriguing in the sense that malaria was endemic in the country (Szénási et al., 2003). The vector capability of a malaria mosquito can also depend on the climate in a region. For example, Anopheles superpictus can transmit both P. vivax and P. falciparum (Aytekin et al., 2009), due to the Mediterranean-Middle Eastern-Central Asian occurrence of this mosquito (Sinka et al., 2012) and the relatively high-temperature threshold of P. falciparum (18 °C compared to the 15 °C threshold of P. vivax; Patz & Olson, 2006). However, An. superpictus is a dominant vector of P. vivax in their occurrence area, not P. falciparum.
It should also be noted that one parasite could also have several vectors depending on the climate, vegetation, and other ecological factors. In the tropical rainforest of Africa, Anopheles funestus and Anopheles gambiae are the main vectors of P. falciparum because these mosquitoes prefer the continuously humid and hot climate of Central Africa (Sinka et al., 2012). The secondary but still important malaria vectors are Anopheles moucheti and Anopheles nili. In the forested savanna environments, An. funestus, An. gambiae, and Anopheles arabiensis are the primary vectors of falciparum malaria. In the savanna-type settings, these are An. funestus and An. arabiensis, and in the Sahel, An. arabiensis plays the role of the primary vector of P. falciparum. In addition, the coastal areas of West, Central, and East Africa have different mosquito assemblages in the case of the secondary vectors. The above-described fact indicates several limitations of host switch on the side of the vectors, the hosts, and the parasites. These facts do not contradict the probability of frequent host switches of Plasmodium species between apes and humans during the evolutionary history of the human species and their relatives.
Materials and Methods
This study follows these steps to understand when and where the host switches of malaria parasites between primates and ancient humans might have occurred in Africa.
-
(1)
It conducts a palaeoenvironmental characterization of Pliocene and Pleistocene habitats where the coevolution of ancient hominins and malaria Plasmodium species could have occurred.
-
(2)
The climatic suitability patterns of the malaria mosquito fauna were modeled for different Pliocene and Quaternary periods.
-
(3)
The climatic determinants of the distribution of the high diversity malaria mosquito (anopheline) fauna and the extant Gorilla genus were determined and modeled for the Greenlandian and the Northgrippian periods.
-
(4)
The modeled diversity values of the anopheline mosquito fauna and the climatic suitability values of Gorilla species were compared (depending on the period) to the occurrence of Pliocene and Pleistocene hominins and mid- and late Pleistocene humans, including the sites of the Lupemban Culture and the Neolithic farmers in Africa.
Climate Data
The climate data of the reference period (1970–2000) was obtained from the WordClim climate database (WorldClim version 2.1; Fick and Hijmans, 2017). The palaeoclimatic data of these other periods were also used: the Greenlandian and Northgrippian parts of the Holocene; three stages of the Pleistocene era: the MIS9 period, the Last Interglacial Period, and the Last Glacial Period; and the Middle Pliocene (mid-Pliocene warm period and the Marine isotope stage M2 period). These palaeoclimatic reconstructions originated from the PaleoClim database (Brown et al., 2018). Table 1 contains the details of the referenced climate models. For modeling purposes, 11 temperature-like and eight precipitation-nature bioclimatic variables were used (Table 2).
Archaeological Site and Gorilla Occurrence Data
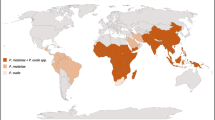
The purpose of this study is to make visible the environment in which ancient people lived as this relates to the host switch probability of malaria Plasmodium species. The Gorilla species is used in the study to represent the great apes since falciparum malaria possibly originated in this genus (Liu et al., 2010).
The sources of the archaeological sites and gorilla distribution data are the following:
-
(1)
Pliocene and the early Pleistocene occurrence of fossil hominin species and the accompanying fossil fauna were gained from the Fossilworks database (Alroy et al., 2018).
-
(2)
The Lupenbam culture-related archaeological site data that represents one of the Middle Stone Age cultures of Central and West Africa were gained from Taylor (2011, 2016).
-
(3)
The Neolithic archaeological data of Africa was based on the publications by Brooks et al. (2009, North Africa), Shoemaker and Davies (2019, East Africa), and Sadr (2003, South Africa).
-
(4)
The present distribution of Gorilla species was based on the publication by McRae and Aronsen (2018; Fig. 1).
Environmental Interpretation of the Fossil Zoological Data
Hadar (Ethiopia), the West and East Turkana region (Kenya), and Chad were selected as sample regions for early hominids. Unlike other parts of the continent, these areas present examples of early to mid-Pliocene, mid-Pliocene, and early Pleistocene ancient hominin-related fossil sites. Supplementary Table 1 contains the essential data about the formation, age, and palaeoenvironments of the collecting areas.
To create a more sensible characterization method related to the dryness/wetness of the ancient taphonomical conditions, the plausible environmental requirements of the fossil species were estimated based on their living relatives in Africa. The classification categories were as follows:
-
(1)
Dry (fully terrestrial) environments ( +): deserts, semi-deserts, rocky environments.
-
(2)
Moderately dry environments (+ +): open woodlands, grasslands, forests, and bushlands (drier mesic environment).
-
(3)
Moderately wet (semi-aquatic) environments (+ + +): floodplains, swamps, marshlands. Gallery forests (wetter mesic environment).
-
(4)
Wet environments (+ + + +): freshwater lakes, rivers, and brackish waters.
Based on land characteristics and environmental patterns, dry terrestrial environments are very favorable to very low malaria prevalence or the total absence of malaria. The mesic sites tend to be linked to unstable malaria environments where mosquito breeding sites are only available in the wet season or in small, demarcated areas. The wetlands could be an area of stable malaria as in the present times where mosquito breeding sites are perennially available (e.g., Kabaria et al., 2016; Midekisa et al., 2014; Wimberly et al., 2021).
Determination of the Climate Range of Malaria Mosquito Distribution
The source of the country-wide mosquito data was the Walter Reed Biosystematics Unit (WRBU) database (Gaffigan et al., 2015). From the database, the country-wide number of anopheline (malaria) mosquitoes was used as an indicator of the possibility of altering the host primate since it can be proposed that in those areas where diverse malaria mosquito fauna exist, the probability of host switch is higher than in areas where the diversity of anopheline mosquitoes is low. Six countries (Russia, Canada, China, USA, Brazil, and Australia), with a total area of 5 + million square kilometers, are excluded from this study because their climatic conditions are too heterogenous to handle as a unit of analysis (Supplementary Fig. 1).
Among the remaining 187-member states of the United Nations plus the two observer states, the anopheline mosquito data was available in the case of 158 countries. Sorting the countries by the number of species, it was found that the species of anopheline mosquitoes is between 0 and 20 for 114 countries, 21–40 for 31 countries, and the diversity of malaria mosquitoes reaches or exceeds 41 species in 13 countries. The five most diverse mosquito fauna can be found in South and Southeast Asian countries (Vietnam: 53, India: 62, Malaysia: 75, Thailand: 76, and Indonesia: 77 species). In addition, high anopheline diversity was also found in some African states (Ethiopia: 41, Cameroon: 42, Tanzania: 42, Angola: 48 malaria mosquito species). A strict criterion was used to determine the climatic limits of the low, medium, and high malaria diversity areas: the lower and the upper quartiles of the bioclimatic values related to the diversity were used as limiting factors (Fig. 2).
However, no distinction was made between individual Gorilla species. The area of Gorilla gorilla diehli, Gorilla gorilla gorilla, Gorilla beringei and Gorilla beringei beringei was handled as a single unit. The absolute lower and upper values of the bioclimatic factors related to the fossil sites were applied in further modeling. Supplementary Table 2 shows the climatic limits of the occurrence of extant Gorilla species.
Model Identification
The extreme climate factors described above were used to numerically model the potential (collective) distribution areas of the high diversity anopheline mosquito fauna and Gorilla species. The appearance of the species is characterized by true or false (0; 1) values. Hence, the area which is determined by the cut limit value of a bioclimatic factor can be written in the following form:
Here, bio N represents the bioclimatic factor identification number. The limitation factor values are climate model-independent. The areas excluded by the limiting factors can be summed, and the following formula describes the aggregated distribution area:
A(bio1…..n) shows the potential distribution area of the studied taxonomic units. The results were displayed as heat maps.
Results
Anopheline Diversity Conditions in the Last 3.3 ky
The modeled results show that Africa’s high diversity of malaria mosquito fauna has notable alterations depending on the actual climatic conditions. In general, the continent’s climate would have been more suitable for malaria mosquitoes in the interglacial than in the glacial periods. In the interglacial periods, diverse anopheline fauna likely inhabited the south coast of West Africa, large areas of Central Africa, and the southeast coast of East Africa. However, during the coolest episodes of the drier and cooler glacial periods, the climatic suitability of the diverse malaria mosquito fauna would have notably decreased in Central Africa. In the Last Glacial Maximum, the southeast corner of West African coastland could be the most important refugia of the diverse anopheline fauna in the continent (Fig. 3).
Pliocene and Early Pleistocene Conditions
Comparing the suitability patterns of the high diversity mosquito fauna and the distribution of the Pliocene hominin sites indicates that Pliocene hominins avoided areas that were especially suitable for the high diversity anopheline mosquito fauna. The most hazardous place for ancient malaria could be the northern part of East Africa. However, this assumption could be valid only for the warm and humid interglacial periods. In the cold period, the mean suitability of the high diversity anopheline fauna was 43%; and in the warm period, it could be 48%. It means that although there is a great difference in the suitability values for the modeled Central African patterns between the cold and warm Pliocene periods, this could not have had a notable difference for Pliocene hominins in terms of the probability of malaria transmission from primates to early hominin species. However, the results also indicate that the areas with a high diversity of anopheline mosquito fauna in the eastern part of Central Africa could be close to early hominins-inhabited regions. In contrast, in Chad and South Africa, the probability of host switch appears low, based on the model values (Fig. 4).
The sediment conditions indicate that Chad’s Pliocene-age hominin fossil sites were originally located in wet floodplain areas. The presence of the ancient Lake Chad and its rivers created various aquatic habitats where several fish, aquatic and semi-aquatic turtles, bird and reptiles, and mammal species existed (Table 3). Based on the plausible environmental requirements of the collected fossil vertebrate taxa, the rivers were surrounded by extensive marshes and swamps. Grassy and woodland areas both existed in the wider area. However, the higher number of semi-arid environment mammals indicates that the climate was unstable, and semi-arid regions existed around the marsh areas. The 12% of the fossil animal taxa refer to dry and semi-dry (including the rocky environments), 39% to moderately wet (e.g., savanna and forest), 22% to wet or semi-aquatic (marshes gallery forests), and 27% to freshwater or, in other words, aquatic habitats (lakes, rivers; Fig. 5). Under similar environmental conditions, the climate suitability of human malaria is high, and malaria’s presence is stable in present-day Africa. Similar conditions currently characterize the floodplain areas of the Niger River in West Africa.
Plio-Pleistocene fossil animal taxa were collected in 13 sites related to Australopithecus afarensis in Hadar, Ethiopia (Johanson et al., 1978). The sediments and the presence of bony fish remains indicate permanent, medium-sized, or large streams or lakes. However, aquatic turtles, crocodiles, and water birds are missing from the fossil materials (Table 4). None of the collected vertebrate taxa could prefer dry and semi-dry environments. The majority, 75% of the taxa, are related to moderately wet environments, and 8–17% could live under semi-aquatic and freshwater aquatic conditions (Fig. 5). The most plausible palaeoenvironment is a drier floodplain surrounded by the mosaics of open grasslands and deciduous tropical forests and shrubland, indicating a tropical savanna (Aw/Af) environment. Under similar conditions, the climate suitability of human malaria is low in the fully terrestrial ecotypes and moderate along the floodplains. The presence of malaria can be unstable in such areas. The floodplains and the surrounding rivers of eastern and southeastern Ethiopia provide similar conditions in the present times (e.g., Awash and Wabi Shebele).
The fossil vertebrate fauna of nine H. erectus-related collecting sites in the East and West Turkana region is presented in Table 5. The environment and the vertebrate fossils indicate a tropical forest-savanna mosaic (Aw/As) crossed by medium-sized rivers that emptied into Lake Turkana. Many of the ancient mammal fauna might have lived in the ecotone between woodlands and savannas or in the forested savanna. However, many hot semi-arid climate (BSh)-tolerating species indicate (Supplementary Table 3) that the area was at the margin of tropical savanna and semi-arid environments, and climate fluctuations could strongly affect the ecology of the Turkana region. Only 21% of the fossil vertebrate taxa could be tied to a dry or semi-dry environment, 71% to moderately wet, and 9% to semi-wet. None is expressly associated with wet environmental conditions (Fig. 5). Due to the proximity of the ancient Turkana Lake and the oxbows, several aquatic and semi-aquatic habitats existed around the lake and in the floodplains. Under similar climatic conditions, the climate suitability of human malaria is unstable, or there is no malaria in the present time.
It can be said that the taphocenosis of the Chad sites refers to a wet palaeoenvironment with the presence of several aquatic and semi-aquatic animal taxa. This environment would have favored the breeding sites of anopheline mosquitoes, and the mosquito populations could be abundant. The fossil assemblages of the Hadar sites refer to drier conditions, but mosquito breeding sites might also have been present in this area. In the case of the Turkana sites, the animal fossils of the ancient hominin-related sites refer to a relatively dry environment in which the malaria mosquito breeding sites could occur only occasionally, plausibly only during the wet season.
Late Pleistocene Conditions
The possibility of a high diversity of anopheline mosquito during the Last Glacial Maximum and the Last Interglacial periods suggests that the Middle Stone Age Lupemban Culture archaeological sites (age: ~ 300–12 ka) would have been in regions where the diversity of malaria mosquito fauna could be medium level or moderately high. However, the conditions could depend on the area and the glacial/interglacial status of the period. The modeled mean suitability values of the highly diverse anopheline fauna in the Lupemban sites are 54% in the Last Glacial Maximum and 71.5% in the Last Interglacial Period. The human populations of the southeast coast of West Africa could permanently live in such regions where the malaria mosquito fauna could be highly diverse on a global level (Fig. 6A–B). In West Africa, the mean suitability values of the diverse malaria mosquito fauna are 64% in the Greenlandian and 66% in the Northgrippian periods. However, the values might have been as high as 97–93% in some areas of West and Central Africa (Fig. 6C–D).
The Last Glacial Maximum’s (A) and the Last Interglacial Period’s (B) models of the high diversity anopheline fauna with the archaeological sites of the Lupemban Culture (Middle Stone Age) in Africa; the Greenlandian (C) and the Northgrippian (D) period’s models of the high diversity anopheline fauna with early agricultural sites in the southern part of West and the northwestern part of Central Africa
Early to Mid-Holocene Conditions
Projecting Later Stone Age sites to the gorilla-related climatic suitability map, it seems that the encounter between human populations and gorillas and the malaria parasites of these apes might have occurred most probably along the coasts of the Guinean Bay in West and Central Africa and maybe in the eastern part of Central Africa (Fig. 7).
Discussion
In mid-Pliocene Africa, ancient humans likely lived in climatic conditions where the suitability of the highly diverse anopheline fauna was moderate in cold and warm periods. The investigation of the fossil assemblages also confirms this hypothesis showing that specific early hominin populations lived near wetlands where mosquito breeding sites also occur. Most of these permanently wet habitats could be linked to tropical wet (Af, Am) climates. Africa’s tropical forest and woodland habitats are areas where malaria is always present (Kibret et al., 2015). These regions include West and Central Africa, the Kongo Basin, certain areas of the East African Rift Valley, and Southeast Africa. For example, in 2000–2019, the malaria incidence was the highest in the southern part of West Africa, Central Africa, and the southern regions of East Africa. The prevalence of sickle cell disease also follows this pattern (Kato et al., 2018), evidence of a long-lasting selection effect against falciparum malaria in these areas.
Several extinct hominoid species are known from the Late Palaeogene period to the end of the Pleistocene epoch in Africa and Eurasia. Presumably, each species may have had its malaria parasite lineages. The oldest fossil malaria mosquito species, Anopheles rottensis, was found in the Upper Oligocene (Chattian) lacustrine strata of the Rott Formation of Germany (Harbach, 2013; Statz, 1944). However, the genus may have been established earlier, presumably sometime in the Upper Eocene epoch. The hominoid clade originated in Africa in the Late Oligocene epoch (Begun, 2007) and their radiation in Eurasia occurred in the turn of the late Early Miocene and the early mid-Miocene epochs (Casanovas-Vilar et al., 2011). This event was linked to the formation of the Gomphotherium land bridge, which connected the Afro-Arabian continent and Eurasia (Harzhauser et al., 2007). For the early Late Miocene epoch, hominoid species populated large areas of Africa and the tropical and subtropical climate regions of Eurasia (Li et al., 2020). It is very likely that the early hominoids already had their Plasmodium parasites because their closest relatives, catarrhine monkeys, also have simian malaria parasites (Eyles, 1963). Even the earliest anthropoids lived in malaria-risk areas. For example, the primitive anthropoid, Aegyptopithecus zeuxis lived in a shallow lake/marsh-like environment and the gallery forest of a very low energy stream in the Fayum area of Egypt during the early part of the Oligocene epoch (Seiffert, 2006).
Several other hominoids also preferred swamp forests and different wetland habitats. For example, the Iberian Hispanopithecus laietanus lived in subtropical marshy and riparian areas (Marmi et al., 2012). The Central European Rudapithecus hungaricus inhabited dense swamp forests (Andrews & Cameron, 2010). Other important Late Miocene hominoid-fossils-bearing sites, including the Central European Mariathal, Götzendorf an der Leitha, and Klein Hadersdorf, were likely localized coastal bald cypress swamp forests (Antalfi & Fehér, 2013; Erdei et al., 2007). All of these indicate that many Late Miocene hominoid populations in Western Eurasia specifically favored wetland habitats, which provided extensive breeding sites and adequate climatic conditions for malaria mosquitoes. Some authors suggested that the evolutionary lineage of the extant African primates and humans is due to a back-migration evolutionary event from Eurasia to Africa in the Latest Miocene epoch (e.g., Begun & Kordos, 1997; Begun & Nargolwalla, 2004; Moya-Sola et al., 2009). If this assumption is valid, it is not impossible that the ancestor of P. vivax, which can perform its extrinsic developmental cycle at relatively low temperatures, may have completed a part of its evolution in the more temperate lands of Western Eurasia.
In the Pliocene and the Pleistocene epochs, the diversity of malaria parasites—including the ancestors of P. vivax and P. falciparum—could have been considerably more significant than it is today. Recent discoveries demonstrate a wide range of hominid species during the last 3 million years before the appearance of modern humans. Appearance of modern humans. Homo erectus, Homo habilis, Homo naledi, Homo bodoensis, and various australopithecines in Africa, as well as Neanderthals, Denisovans, and other ancient humans outside the continent, may have been exposed to both malaria parasites and their vectors. Middle Palaeolithic archaeological sites in Eurasia are often associated with wetland-margin environments, swamps, and seasonal riverbeds. These environments are the most preferred natural breeding habitats of competent anopheline malaria vectors (Ondiba et al., 2019). For example, wetland-associated Middle Palaeolithic sites were found at the Shishan Marsh in Jordan (Pokines et al., 2019), in the Schöningen open pit mine in Germany (Turner et al., 2018), in the ancient lakeshore sites of the Nefud Desert, Saudi Arabia (Petraglia et al., 2011), and the Eastern Desert of Egypt (Kindermann et al., 2018). It is plausible that the network of these Middle Palaeolithic sites was influenced by ancient malaria prevalence patterns (Trájer et al., 2020).
It seems that the host switch of malaria parasites between great apes and humans occurred several times, even in the early stages of Hominidae evolution in Africa. A similar multiple host switches event was also suggested in the case of P. vivax (Mu et al., 2005). Furthermore, lateral switches between distantly related hosts of malaria parasites may also have occurred (Garamszegi, 2009). It implies that the transfer of Plasmodium species could occur between apes and humans and between old-world monkeys and humans. Most of the studies, which deal with a human evolution ancient malaria, focus on the origin of the extant malaria species and the possibility of the descent of different Plasmodium lineages. However, while anatomically modern humans lived at least as early as 286 kya (Richter et al., 2017), human populations in about 70 kya underwent a bottleneck event (Ambrose, 2003). The decrease in the population of human hosts might have substantially reduced the diversity or caused the extinction of some malaria parasites in Africa. This could explain why the human hemoglobin sickle cell mutation is so young, ca. 22 ky old (Laval et al., 2019). Based on the model results, several Lupemban sites are close to the areas with diverse malaria mosquito fauna in West and Central Africa. This indicates that such competent malaria vectors like An. gambiae have the chance and a long time to adapt to the blood of humans. Interestingly, about 50 kya, an unknown archaic human group interbred with modern humans in Africa, with 2–19% of this archaic population’s genetic ancestry being present in modern African human populations (Durvasula & Sankararaman, 2020).
During the Pleistocene era in Africa, the coastal areas of the Gulf of Guinea could be the most stable for the high diversity of anopheline fauna. Likely, the host switch of a Laverania species and its transformation to the present-day P. falciparum parasite in humans occurred along the coasts of the Gulf of Guinean, between Sierra-Leone and Gabon, in the transition zone of the tropical monsoon and tropical savanna climate areas, or the northeastern parts of Central Africa in present-day Burundi, Rwanda, and Uganda. Coluzzi (1999) and Rich et al. (2009) hypothesized that the anthropophilic taxa of Afrotropical malaria mosquitoes (An. funestus and An. gambiae complexes) emerged in the Neolithic period parallel to the increase of human populations due to economic and demographic consequences of agricultural innovations. The present-day highly aggressive falciparum malaria has a high prevalence in Central Africa. However, it is interesting that the rainforest hunter-gatherers of equatorial Africa acquired the βS mutation from agriculturalists through adaptive gene flow only in the last 6 ky (Laval et al., 2019), indicating that the equatorial regions could not have been the source of malignant malaria. The results of this study suggest that considering both the long-term climatic suitability patterns of the diverse anopheline mosquito faunae, the occurrence of Gorilla species, and the ways of agricultural beginnings and expansion, two main foci of malaria parasite-host switch could have existed in Africa: West-Central Africa and the East African Rift Valley territories of the continent. However, it cannot be ruled out that some human malaria parasite lineages survived this period and still exist in great apes or other ancient humans. Since the Anderson-May model of host-parasite dynamics states that infections of different levels of virulence become extinct if they do not optimize the basic reproductive rate of the causative parasite (Bremermann & Thieme, 1989), it can be hypothesized that higher virulence and greater mortality rates would have occurred in larger and well-connected human populations than in small and relatively isolated populations. This reasoning can explain why malignant malaria lineages might have arrived in tropical Africa with the mobile and populous farming groups.
The hypothesis about the origin of falciparum malaria is consistent with the results of the study by Mu et al. (2002). They found that even the lineage of the ancestor of the falciparum parasite could be much older. It may have appeared during the Last Interglacial Period, but the extant P. falciparum underwent a severe population bottleneck about 3–6 ky ago. It is likely then that the encounter of the ancestor of P. falciparum and human populations occurred in West, North-Central Africa, or East Africa, where the migrating farming populations reached the area of the present-day south Sahel belt about 7–5 kya. Although a genetic study found that the paternal lineages of most North Africans emerged 15 ka and the population splits started after the desiccation of the Sahara (Vai et al., 2019), Haber et al. (2016) suggested that multiple Holocene Eurasian migrations also marked the genetic history of Africa ca. 7.2–3.0 kya. These demographic movements strongly affected the genetic patterns of North Africa.
Conclusions
The host switch of primates-related malaria species could have happened several times in the last 5.3 my in Africa. However, certain permanent patterns can be observed. Great apes such as the Gorilla species lived in those regions where the malaria mosquito diversity could be very high. In contrast, the known archaeological sites of ancient hominins can be found in moderately diverse regions. West-Central and West Africa, as well as certain regions of East-Central Africa, could be the areas where the host switch of Plasmodium species between the great apes and hominins took place.
References
Alroy, J., Uhen, M. D., Behrensmeyer, A. K., Turner, A., Jaramillo, C., Mannion, P., et al. (2018). Taxonomic occurrences of Mammalia recorded in Fossilworks, the 555 Evolution of Terrestrial Ecosystems database, and the Paleobiology Database. 556 Fossilworks.
Ambrose, S. H. (2003). Did the super-eruption of Toba cause a human population bottleneck? Reply to Gathorne-Hardy and Harcourt-Smith. Journal of Human Evolution, 45(3), 231–237.
Andrews, P., & Cameron, D. (2010). Rudabànya: Taphonomic analysis of a fossil hominid site from Hungary. Palaeogeography, Palaeoclimatology, Palaeoecology, 297(2), 311–329. https://doi.org/10.1016/j.palaeo.2010.08.010
Angel, J. L. (1966). Porotic hyperostosis, anemias, malarias and marshes in the prehistoric Mediterranean. Science, 153(3737), 760–763. https://doi.org/10.1126/science.153.3737.760
Annan, Z., Durand, P., Ayala, F. J., Arnathau, C., Awono-Ambene, P., Simard, F., et al. (2007). Population genetic structure of Plasmodium falciparum in the two main African vectors, Anopheles gambiae and Anopheles funestus. Proceedings of the National Academy of Sciences, 104(19), 7987–7992. https://doi.org/10.1073/pnas.0702715104
Antalfi, E., & Fehér, S. (2013). Xylotomic similarities and natural habitat of the fossil remains of Bükkábrány. Acta Biologica Szegediensis, 57(2), 161–166.
Aytekin, S., Aytekin, A. M., & Alten, B. (2009). Effect of different larval rearing temperatures on the productivity (Ro) and morphology of the malaria vector Anopheles superpictus Grassi (Diptera: Culicidae) using geometric morphometrics. Journal of Vector Ecology, 34(1), 32–42. https://doi.org/10.1111/j.1948-7134.2009.00005.x
Begun, D. R. (2007). Fossil record of Miocene hominoids. Handbook of Paleoanthropology, 2, 921–977. https://doi.org/10.1007/978-3-642-39979-4_32
Begun, D. R., & Kordos, L. (1997). Phyletic affinities and functional convergence in Dryopithecus and other Miocene and living hominids. In Begun, D. R., Ward, C. V., Rose, M. D. (Eds), Function, phylogeny, and fossils. Advances in Primatology. Springer, Boston, MA. https://doi.org/10.1007/978-1-4899-0075-3_14
Begun, D. R., & Nargolwalla, M. C. (2004). Late Miocene hominid biogeography: Some recent perspectives. Evolutionary Anthropology, 13(6), 234–238. https://doi.org/10.1002/evan.20025
Bobe, R., Manthi, F. K., Ward, C. V., Plavcan, J. M., & Carvalho, S. (2020). The ecology of Australopithecus anamensis in the early Pliocene of Kanapoi. Kenya. Journal of Human Evolution, 140(2020), 102717. https://doi.org/10.1016/j.jhevol.2019.102717
Bremermann, H. J., & Thieme, H. R. (1989). A competitive exclusion principle for pathogen virulence. Journal of Mathematical Biology, 27(2), 179–190. https://doi.org/10.1007/BF00276102
Brooks, N., Clarke, J., Garfi, S., & Pirie, A. (2009). The archaeology of Western Sahara: Results of environmental and archaeological reconnaissance. Antiquity, 83(322), 918–934. https://doi.org/10.1017/S0003598X00099257
Brown, J. L., Hill, D. J., Dolan, A. M., Carnaval, A. C., & Haywood, A. M. (2018). PaleoClim, high spatial resolution paleoclimate surfaces for global land areas. Scientific Data, 5(1), 1–9. https://doi.org/10.1038/sdata.2018.254
Casanovas-Vilar, I., Alba, D. M., Garcés, M., Robles, J. M., & Moyà-Solà, S. (2011). Updated chronology for the Miocene hominoid radiation in Western Eurasia. Proceedings of the National Academy of Sciences, 108(14), 5554–5559. https://doi.org/10.1073/pnas.1018562108
Coluzzi, M. (1999). The clay feet of the malaria giant and its African roots: Hypotheses and inferences about origin, spread and control of Plasmodium falciparum. Parassitologia, 41(1–3), 277–283.
Costa, M. A., Matheson, C., Iachetta, L., Llagostera, A., & Appenzeller, O. (2009). Ancient leishmaniasis in a highland desert of northern Chile. PLoS ONE, 4(9), e6983. https://doi.org/10.1371/journal.pone.0006983
Daron, J., Boissiere, A., Boundenga, L., Ngoubangoye, B., Houze, S., Arnathau, C., Sidobre, C., et al. (2020). Population genomic evidence of a Southeast Asian origin of Plasmodium vivax. Science Advances, 7(18), eabc3713. https://doi.org/10.1126/sciadv.abc3713
de Ruiter, D. J., Sponheimer, M., & Lee-Thorp, J. A. (2008). Indications of habitat association of Australopithecus robustus in the Bloubank Valley, South Africa. Journal of Human Evolution, 55(6), 1015–1030. https://doi.org/10.1016/j.jhevol.2008.06.003
Domínguez-Rodrigo, M., Pickering, T. R., Diez-Martín, F., Mabulla, A., Musiba, C., Trancho, G., Baquedano, E., et al. (2012). Earliest porotic hyperostosis on a 15-million-year-old hominin, Olduvai Gorge. Tanzania. Plos One, 7(10), 46414. https://doi.org/10.1371/journal.pone.0046414
Durvasula, A., & Sankararaman, S. (2020). Recovering signals of ghost archaic introgression in African populations. Science Advances, 6(7), eaax5097.
Erdei, B., Hably, L., Kázmér, M., Utescher, T., & Bruch, A. A. (2007). Neogene flora and vegetation development of the Pannonian domain in relation to palaeoclimate and palaeogeography. Palaeogeography, Palaeoclimatology, Palaeoecology, 253(1–2), 115–140. https://doi.org/10.1016/j.palaeo.2007.03.036
Eyles, D. E. (1963). The species of simian malaria: Taxonomy, morphology, life cycle, and geographical distribution of the monkey species. The Journal of Parasitology, 49(6), 866–887.
Fecchio, A., Bell, J. A., Collins, M. D., Farias, I. P., Trisos, C. H., Tobias, J. A., et al. (2018). Diversification by host switching and dispersal shaped the diversity and distribution of avian malaria parasites in Amazonia. Oikos, 127(9), 1233–1242. https://doi.org/10.1111/oik.05115
Fick, S. E., & Hijmans, R. J. (2017). WorldClim 2: New 1km spatial resolution climate surfaces for global land areas. International Journal of Climatology, 37(12), 4302–4315. https://doi.org/10.1002/joc.5086
Field, D. J. (2020). Preliminary paleoecological insights from the Pliocene avifauna of Kanapoi, Kenya: Implications for the ecology of Australopithecus anamensis. Journal of Human Evolution, 140(2020), 102384. https://doi.org/10.1016/j.jhevol.2017.08.007
Fillios, M., Field, J., & Charles, B. (2010). Investigating human and megafauna co-occurrence in Australian prehistory: Mode and causality in fossil accumulations at Cuddie Springs. Quaternary International, 211(1–2), 123–143. https://doi.org/10.1016/j.quaint.2009.04.003
Flint, J., Hill, A. V. S., Bowden, D. K., Oppenheimer, S. J., Sill, P. R., Serjeantson, S. W., et al. (1986). High frequencies of α-thalassaemia are the result of natural selection by malaria. Nature, 321(6072), 744–750. https://doi.org/10.1038/321744a0
Gaffigan, T. V., Wilkerson, R. C., Pecor, J. E., Stoffer, J. A., & Anderson, T. (2015). Systematic catalog of Culicidae. Walter Reed Biosystematics Unit. http://www.mosquitocatalog.org/. [Acessed January 2021].
Garamszegi, L. Z. (2009). Patterns of co-speciation and host switching in primate malaria parasites. Malaria Journal, 8(1), 1–15. https://doi.org/10.1186/1475-2875-8-110
Gerszten, E., Allison, M. J., & Maguire, B. (2012). Paleopathology in South American mummies: A review and new findings. Pathobiology, 79(5), 247–256. https://doi.org/10.1159/000334087
Gomes, E. C. D. S., Cruz, D. L. D., Santos, M. A. V. M., Souza, R. M. C., Oliveira, C. M. F. D., Ayres, C. F. J., et al. (2020). Outbreak of autochthonous cases of malaria in coastal regions of Northeast Brazil: The diversity and spatial distribution of species of Anopheles. Parasites & Vectors, 13(1), 1–11. https://doi.org/10.1186/s13071-020-04502-7
Gowland, R. L., & Western, A. G. (2012). Morbidity in the marshes: Using spatial epidemiology to investigate skeletal evidence for malaria in Anglo-Saxon England (AD 410–1050). American Journal of Physical Anthropology, 147(2), 301–311. https://doi.org/10.1002/ajpa.21648
Haber, M., Mezzavilla, M., Bergström, A., Prado-Martinez, J., Hallast, P., Saif-Ali, R., et al. (2016). Chad genetic diversity reveals an African history marked by multiple Holocene Eurasian migrations. The American Journal of Human Genetics, 99(6), 1316–1324. https://doi.org/10.1016/j.ajhg.2016.10.012
Harbach, R. E. (2013). The phylogeny and classification of Anopheles. In S. Manguin (Ed.), Anopheles mosquitoes: New insights into malaria vectors. (pp. 3–55), https://doi.org/10.5772/54695
Harzhauser, M., Latal, C., & Piller, W. E. (2007). The stable isotope archive of Lake Pannon as a mirror of Late Miocene climate change. Palaeogeography, Palaeoclimatology, Palaeoecology, 249(3–4), 335–350. https://doi.org/10.1016/j.palaeo.2007.02.006
Henry, A. G., Ungar, P. S., Passey, B. H., Sponheimer, M., Rossouw, L., Bamford, M., et al. (2012). The diet of Australopithecus sediba. Nature, 487(7405), 90–93. https://doi.org/10.1038/nature11185
Howes, R. E., Reiner, R. C., Jr., Battle, K. E., Longbottom, J., Mappin, B., Ordanovich, D., et al. (2015). Plasmodium vivax transmission in Africa. PLoS Neglected Tropical Diseases, 9(11), e0004222.
Kabaria, C. W., Molteni, F., Mandike, R., Chacky, F., Noor, A. M., Snow, R. W., et al. (2016). Mapping intra-urban malaria risk using high resolution satellite imagery: A case study of Dar es Salaam. International Journal of Health Geographics, 15(1), 1–12. https://doi.org/10.1186/s12942-016-0051-y
Kariuki, S. N., & Williams, T. N. (2020). Human genetics and malaria resistance. Human Genetics, 139(6), 801–811. https://doi.org/10.1007/s00439-020-02142-6
Kato, G. J., Piel, F. B., Reid, C. D., Gaston, M. H., Ohene-Frempong, K., Krishnamurti, L., Smith, W. R., et al. (2018). Sickle cell disease. Nature Reviews Disease Primers, 4(1), 1–22. https://doi.org/10.1038/nrdp.2018.10
Kibret, S., Lautze, J., McCartney, M., Wilson, G. G., & Nhamo, L. (2015). Malaria impact of large dams in sub-Saharan Africa: Maps, estimates and predictions. Malaria Journal, 14(1), 1–12. https://doi.org/10.1186/s12936-015-0873-2
Kigen, C. (2019). In silico prediction of anti-malarial activity and pharmacokinetic properties of herbal derivatives of Ajuga remota and Azadirachta indica. B.Sc. thesis. Jomo Kenyatta University of Agriculture and Technology, Nairobi, Kenya.
Kimbel, W. H., Lockwood, C. A., Ward, C. V., Leakey, M. G., Rak, Y., & Johanson, D. C. (2006). Was Australopithecus anamensis ancestral to A afarensis? A case of anagenesis in the hominin fossil record. Journal of Human Evolution, 51(2), 134–152. https://doi.org/10.1016/j.jhevol.2006.02.003
Kindermann, K., Van Peer, P., & Henselowsky, F. (2018). At the lakeshore—An Early Nubian Complex site linked with lacustrine sediments (Eastern Desert, Egypt). Quaternary International, 485(2018), 131–139. https://doi.org/10.1016/j.quaint.2017.11.006
Lalremruata, A., Ball, M., Bianucci, R., Welte, B., Nerlich, A. G., Kun, J. F., et al. (2013). Molecular identification of falciparum malaria and human tuberculosis co-infections in mummies from the Fayum depression (Lower Egypt). PLoS ONE, 8(4), e60307. https://doi.org/10.1371/journal.pone.0060307
Larson, B. (2019). Origin of two most virulent agents of human malaria: Plasmodium falciparum and Plasmodium vivax. In F. H. Kasenga (Ed.), Malaria, (Vol. 4, pp. 1–16). Intech Open Book Series.
Laval, G., Peyrégne, S., Zidane, N., Harmant, C., Renaud, F., Patin, E., et al. (2019). Recent adaptive acquisition by African rainforest hunter-gatherers of the late Pleistocene sickle-cell mutation suggests past differences in malaria exposure. The American Journal of Human Genetics, 104(3), 553–561. https://doi.org/10.1016/j.ajhg.2019.02.007
Lazaridis, I., Nadel, D., Rollefson, G., Merrett, D. C., Rohland, N., Mallick, S., et al. (2016). Genomic insights into the origin of farming in the ancient Near East. Nature, 536(7617), 419–424. https://doi.org/10.1038/nature19310
Li, P., Zhang, C., Kelley, J., Deng, C., Ji, X., Jablonski, N. G., et al. (2020). Late Miocene climate cooling contributed to the disappearance of hominoids in Yunnan region, southwestern China. Geophysical Research Letters, 47(11), e2020GL087741. https://doi.org/10.1029/2020GL087741
Liu, W., Li, Y., Learn, G. H., Rudicell, R. S., Robertson, J. D., Keele, B. F., et al. (2010). Origin of the human malaria parasite Plasmodium falciparum in gorillas. Nature, 467(7314), 420–425. https://doi.org/10.1038/nature09442
Loy, D. E., Liu, W., Li, Y., Learn, G. H., Plenderleith, L. J., Sundararaman, S. A., et al. (2017). Out of Africa: Origins and evolution of the human malaria parasites Plasmodium falciparum and Plasmodium vivax. International Journal for Parasitology, 47(2–3), 87–97. https://doi.org/10.1016/j.ijpara.2016.05.008
Loy, D. E., Plenderleith, L. J., Sundararaman, S. A., Liu, W., Gruszczyk, J., Chen, Y. J., et al. (2018). Evolutionary history of human Plasmodium vivax revealed by genome-wide analyses of related ape parasites. Proceedings of the National Academy of Sciences, 115(36), E8450–E8459. https://doi.org/10.1073/pnas.1810053115
Luzzatto, L. (2012). Sickle cell anaemia and malaria. Mediterranean Journal of Hematology and Infectious Diseases, 4(1), e2012065. https://doi.org/10.4084/mjhid.2012.065
Marciniak, S., Prowse, T. L., Herring, D. A., Klunk, J., Kuch, M., Duggan, A. T., et al. (2016). Plasmodium falciparum malaria in 1st–2nd century CE southern Italy. Current Biology, 26(23), R1220–R1222. https://doi.org/10.1016/j.cub.2016.10.016
Marmi, J., Casanovas-Vilar, I., Robles, J. M., Moyà-Solà, S., & Alba, D. M. (2012). The paleoenvironment of Hispanopithecus laietanus as revealed by paleobotanical evidence from the Late Miocene of Can Llobateres 1 (Catalonia, Spain). Journal of Human Evolution, 62(3), 412–423. https://doi.org/10.1016/j.jhevol.2011.12.003
Martinsen, E. S., Perkins, S. L., & Schall, J. J. (2008). A three-genome phylogeny of malaria parasites (Plasmodium and closely related genera): Evolution of life-history traits and host switches. Molecular Phylogenetics and Evolution, 47(1), 261–273. https://doi.org/10.1016/j.ympev.2007.11.012
McRae, R., & Aronsen, G. P. (2018). Inventory and assessment of the Gorilla gorilla (Savage, 1847) skeletal collection housed at the Yale Peabody Museum of Natural History. Bulletin of the Peabody Museum of Natural History, 59(2), 199–247. https://doi.org/10.3374/014.059.0205
Meyer, M., Arsuaga, J., de Filippo, C., & Nagel, S. (2016). Nuclear DNA sequences from the Middle Pleistocene Sima de los Huesos hominins. Nature, 531(7595), 504–507. https://doi.org/10.1038/nature17405
Midekisa, A., Senay, G. B., & Wimberly, M. C. (2014). Multisensor earth observations to characterize wetlands and malaria epidemiology in Ethiopia. Water Resources Research, 50(11), 8791–8806. https://doi.org/10.1002/2014WR015634
Moyà-Solà, S., Alba, D. M., Almécija, S., Casanovas-Vilar, I., Köhler, M., De Esteban-Trivigno, S., et al. (2009). A unique Middle Miocene European hominoid and the origins of the great ape and human clade. Proceedings of the National Academy of Sciences, 106(24), 9601–9606. https://doi.org/10.1073/pnas.0811730106
Mu, J., Duan, J., Makova, K. D., Joy, D. A., Huynh, C. Q., Branch, O. H., Li, W.-H., et al. (2002). Chromosome-wide SNPs reveal an ancient origin for Plasmodium falciparum. Nature, 418(6895), 323–324. https://doi.org/10.1038/nature00836
Mu, J., Joy, D. A., Duan, J., Huang, Y., Carlton, J., Walker, J., et al. (2005). Host switch leads to emergence of Plasmodium vivax malaria in humans. Molecular Biology and Evolution, 22(8), 1686–1693. https://doi.org/10.1093/molbev/msi160
Multini, L. C., de Souza, A. L. D. S., Marrelli, M. T., & Wilke, A. B. B. (2020). The influence of anthropogenic habitat fragmentation on the genetic structure and diversity of the malaria vector Anopheles cruzii (Diptera: Culicidae). Scientific Reports, 10(1), 1–13. https://doi.org/10.1038/s41598-020-74152-3
Obertová, Z., & Thurzo, M. (2008). Relationship between cribra orbitalia and enamel hypoplasia in the early medieval Slavic population at Borovce. Slovakia. International Journal of Osteoarchaeology, 18(3), 280–292. https://doi.org/10.1002/oa.937
Ondiba, I. M., Oyieke, F. A., Athinya, D. K., Nyamongo, I. K., & Estambale, B. B. (2019). Larval species diversity, seasonal occurrence and larval habitat preference of mosquitoes transmitting Rift Valley fever and malaria in Baringo County. Kenya. Parasites & Vectors, 12(1), 1–14. https://doi.org/10.1186/s13071-019-3557-x
Patz, J. A., & Olson, S. H. (2006). Malaria risk and temperature: Influences from global climate change and local land use practices. Proceedings of the National Academy of Sciences, 103(15), 5635–5636. https://doi.org/10.1073/pnas.0601493103
Petraglia, M. D., Alsharekh, A. M., Crassard, R., Drake, N. A., Groucutt, H., Parker, A. G., et al. (2011). Middle Paleolithic occupation on a marine isotope stage 5 lakeshore in the Nefud Desert. Saudi Arabia. Quaternary Science Reviews, 30(13–14), 1555–1559. https://doi.org/10.1016/j.quascirev.2011.04.006
Pokines, J. T., Lister, A. M., Ames, C. J., Nowell, A., & Cordova, C. E. (2019). Faunal remains from recent excavations at Shishan Marsh 1 (SM1), a Late Lower Paleolithic open-air site in the Azraq Basin. Jordan. Quaternary Research, 91(2), 768–791. https://doi.org/10.1017/qua.2018.113
Reynolds, S. C., Bailey, G. N., & King, G. C. (2011). Landscapes and their relation to hominin habitats: Case studies from Australopithecus sites in eastern and southern Africa. Journal of Human Evolution, 60(3), 281–298. https://doi.org/10.1016/j.jhevol.2010.10.001
Rich, S. M., Leendertz, F. H., Xu, G., LeBreton, M., Djoko, C. F., Aminake, M. N., et al. (2009). The origin of malignant malaria. Proceedings of the National Academy of Sciences, 106(35), 14902–14907. https://doi.org/10.1073/pnas.0907740106
Richter, D., Grün, R., Joannes-Boyau, R., Steele, T. E., Amani, F., Rué, M., et al. (2017). The age of the hominin fossils from Jebel Irhoud, Morocco, and the origins of the Middle Stone Age. Nature, 546(7657), 293–296. https://doi.org/10.1038/nature22335
Robson, A. J., Petty, J., Joyce, D. C., Marques, J. R., & Hofman, P. J. (2014). High resolution remote sensing, GIS and Google Earth for avocado fruit quality mapping and tree number auditing. In 29th International Horticultural Congress on Horticulture Sustaining Lives Livelihoods and Landscapes (IHC2014), 1130(2014), 589–596. https://doi.org/10.17660/ActaHortic.2016.1130.88
Sadr, K. (2003). The Neolithic of southern Africa. The Journal of African History, 44(2), 195–209. https://doi.org/10.1017/S0021853702008393
Seiffert, E. R. (2006). Revised age estimates for the later Paleogene mammal faunas of Egypt and Oman. Proceedings of the National Academy of Sciences, 103(13), 5000–5005. https://doi.org/10.1073/pnas.0600689103
Shoemaker, A., & Davies, M. I. (2019). Grinding-stone implements in the eastern African Pastoral Neolithic. Azania: Archaeological Research in Africa, 54(2), 203–220. https://doi.org/10.1080/0067270X.2019.1619284
Silva, J. C., Egan, A., Friedman, R., Munro, J. B., Carlton, J. M., & Hughes, A. L. (2011). Genome sequences reveal divergence times of malaria parasite lineages. Parasitology, 138(13), 1737–1749. https://doi.org/10.1017/S0031182010001575
Sinka, M. E., Bangs, M. J., Manguin, S., Rubio-Palis, Y., Chareonviriyaphap, T., Coetzee, M., et al. (2012). A global map of dominant malaria vectors. Parasites & Vectors, 5(1), 1–11. https://doi.org/10.1186/1756-3305-5-69
Statz, G. (1944). Neue Dipteren (Nematocera) aus dem Oberoligozän von Rott. II. Familie: Fungivoridae (Pilzmücken). Palaeontographica Abteilung A Band A095 Lieferung, 3–6(1944), 67–92.
Sundararaman, S. A., Plenderleith, L. J., Liu, W., Loy, D. E., Learn, G. H., Li, Y., et al. (2016). Genomes of cryptic chimpanzee Plasmodium species reveal key evolutionary events leading to human malaria. Nature Communications, 7(1), 1–14. https://doi.org/10.1038/ncomms11078
Szénási, Z., Vass, A., Melles, M., Kucsera, I., Danka, J., Csohán, A., & Krisztalovics, K. (2003). Malaria in Hungary: Origin, current state and principles of prevention. Orvosi Hetilap, 144(21), 1011–1018.
Tachibana, S. I., Kawai, S., Katakai, Y., Takahashi, H., Nakade, T., Yasutomi, Y., et al. (2015). Contrasting infection susceptibility of the Japanese macaques and cynomolgus macaques to closely related malaria parasites, Plasmodium vivax and Plasmodium cynomolgi. Parasitology International, 64(3), 274–281. https://doi.org/10.1016/j.parint.2014.10.004
Takemoto, H., Kawamoto, Y., & Furuichi, T. (2015). How did bonobos come to range south of the Congo River? Reconsideration of the divergence of Pan paniscus from other Pan populations. Evolutionary Anthropology: Issues, News, and Reviews, 24(5), 170–184. https://doi.org/10.1002/evan.21456
Taylor, N. (2016). Across rainforests and woodlands: A systematic reappraisal of the Lupemban Middle Stone Age in Central Africa. In S. Jones and B. Stewart (Eds.), Africa from MIS 6–2. Vertebrate Paleobiology and Paleoanthropology. Springer. https://doi.org/10.1007/978-94-017-7520-5_15
Taylor, N. (2011). The origins of hunting and gathering in the Congo basin: A perspective on the Middle Stone Age Lupemban industry. Before Farming, 2011(1), 1–20.
Theodorakopoulou, K., & Karamanou, M. (2020). Human paleopathology during the stone age. Archives of the Balkan Medical Union, 55(4), 11–18. https://doi.org/10.31688/ABMU.2020.55.4.15
Trájer, A. J. (2018). Which mosquitoes (Diptera: Culicidae) are candidates for DNA extraction in forensic practice? Journal of Forensic and Legal Medicine, 58(2018), 183–191. https://doi.org/10.1016/j.jflm.2018.07.002
Trájer, A. J. (2021). The potential persistence of ancient malaria through the Quaternary period in Europe. Quaternary International, 586(2021), 1–13. https://doi.org/10.1016/j.quaint.2021.02.014
Trájer, A. J., Sebestyén, V., & Domokos, E. (2020). The potential impacts of climate factors and malaria on the Middle Palaeolithic population patterns of ancient humans. Quaternary International, 565(2020), 94–108. https://doi.org/10.1016/j.quaint.2020.10.056
Turner, E., Hutson, J., Villaluenga, A., García Moreno, A., & Gaudzinski-Windheuser, S. (2018). Bone staining in waterlogged deposits: A preliminary contribution to the interpretation of near-shore find accumulation at the Schöningen 13II-4 ‘Spear-Horizon’ site, Lower Saxony. Germany. Historical Biology, 30(6), 767–773. https://doi.org/10.1080/08912963.2017.1334203
Twohig, K. A., Pfeffer, D. A., Baird, J. K., Price, R. N., Zimmerman, P. A., Hay, S. I., et al. (2019). Growing evidence of Plasmodium vivax across malaria-endemic Africa. PLoS Neglected Tropical Diseases, 13(1), e0007140.
Vai, S., Sarno, S., Lari, M., Luiselli, D., Manzi, G., Gallinaro, M., et al. (2019). Ancestral mitochondrial N lineage from the Neolithic ‘green’ Sahara. Scientific Reports, 9(1), 1–9. https://doi.org/10.1038/s41598-019-39802-1
Villaseñor, A., Bobe, R., & Behrensmeyer, A. K. (2020). Middle Pliocene hominin distribution patterns in Eastern Africa. Journal of Human Evolution, 147, 102856. https://doi.org/10.1016/j.jhevol.2020.102856
Ward, C., Leakey, M., & Walker, A. (1999). The new hominid species Australopithecus anamensis. Evolutionary Anthropology: Issues, News, and Reviews, 7(6), 197–205.
WHO (2020): World Health Organization. (2020). World malaria report 2020: 20 years of global progress and challenges. URL: https://www.who.int/publications/i/item/9789240015791 (Accessed on 22 December 2021).
Wimberly, M. C., de Beurs, K. M., Loboda, T. V., & Pan, W. K. (2021). Satellite observations and malaria: New opportunities for research and applications. Trends in Parasitology, 37(6), 525–537. https://doi.org/10.1016/j.pt.2021.03.003
Zink, A. R., Spigelman, M., Schraut, B., Greenblatt, C. L., Nerlich, A. G., & Donoghue, H. D. (2006). Leishmaniasis in ancient Egypt and upper Nubia. Emerging Infectious Diseases, 12(10), 1616–1617. https://doi.org/10.3201/eid1210.060169
Funding
Open access funding is provided by University of Pannonia. The research presented in this article was carried out with the financial support of the NKFIH-471–3/2021 project of the Hungarian Ministry for Innovation and Technology.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The author declares no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Trájer, A.J. The Possible Time and Region of Host Switches of Ancient Malaria Parasites with Reference to the Pliocene–Quaternary Archaeological Sites in Africa. Afr Archaeol Rev 39, 283–302 (2022). https://doi.org/10.1007/s10437-022-09483-9
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10437-022-09483-9