Abstract
Background
The standard treatment for unresectable advanced/recurrent esophageal cancer in Japan is 5-fluorouracil plus platinum-containing drugs as first-line chemotherapy and taxanes as second-line chemotherapy. However, the standard regimen after patients become refractory to these treatments remains to be established. Therefore, we investigated the efficacy of trifluridine/tipiracil (FTD/TPI) in patients with esophageal cancer who are refractory or intolerant to 5-fluorouracil, platinum-containing drugs, and taxanes.
Methods
This single-arm phase II trial was conducted in seven hospitals in Japan. Eligible patients were those with unresectable advanced/recurrent esophageal cancer that was refractory or intolerant to 5-fluorouracil, platinum-containing drugs, and taxanes. The primary endpoint was the 3-month progression-free survival rate, and the secondary endpoints were the 6-month progression-free survival rate, progression-free survival, overall survival, response rate, disease control rate, and toxicity.
Results
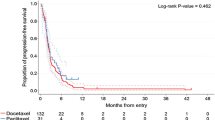
Forty-two patients were enrolled between October 2015 and June 2016. All tumors were squamous cell carcinomas. The progression-free survival rates at 3 and 6 months were 15.4% (90% confidence interval 7.4–26.0%) and 7.7% (90% confidence interval 2.6–16.6%), respectively. The median progression-free survival and median overall survival were 1.3 (95% confidence interval 1.0–1.8) months and 4.5 (95% confidence interval 3.6–5.7) months, respectively. The response rate was 0%, and the disease control rate was 23.8% (95% confidence interval 13.5–38.5%). The major grade 3/4 toxicities were neutropenia (47.6%), leukocytopenia (35.7%), and anemia (21.4%). No treatment-related deaths occurred. Exploratory subgroup analyses showed better progression-free survival in the subgroup without distant metastasis at diagnosis.
Conclusions
Trifluridine/tipiracil monotherapy is feasible and shows modest activity in patients with refractory esophageal squamous cell carcinoma.




Similar content being viewed by others
References
Global Burden of Disease Collaboration, Fitzmaurice C, Abate D, Abbasi N, et al. Global, regional, and national cancer incidence, mortality, years of life lost, years lived with disability, and disability-adjusted life-years for 29 cancer groups, 1990 to 2017: a systematic analysis for the Global Burden of Disease study. JAMA Oncol. 2019;5:1749–68.
Foundation for Promotion of Cancer Research. Cancer statistics in Japan 2019. https://ganjoho.jp/reg_stat/statistics/brochure/backnumber/2019_jp.html. Accessed 21 Jun 2021
Watanabe M, Tachimori Y, Oyama T, et al. Comprehensive registry of esophageal cancer in Japan, 2013. Esophagus. 2021;18:1–24.
Hayashi K, Ando N, Watanabe H, et al. Phase II evaluation of protracted infusion of cisplatin and 5-fluorouracil in advanced squamous cell carcinoma of the esophagus: a Japan Esophageal Oncology Group (JEOG) Trial (JCOG9407). Jpn J Clin Oncol. 2001;31:419–23.
Cooper JS, Guo MD, Herskovic A, et al. Chemoradiotherapy of locally advanced esophageal cancer: long-term follow-up of a prospective randomized trial (RTOG 85–01). Radiation Therapy Oncology Group. JAMA. 1999;281:1623–7.
Iizuka T, Kakegawa T, Ide H, et al. Phase II evaluation of cisplatin and 5-fluorouracil in advanced squamous cell carcinoma of the esophagus: a Japanese Esophageal Oncology Group Trial. Jpn J Clin Oncol. 1992;22:172–6.
Anderson SE, O’Reilly EM, Kelsen DP, et al. Phase II trial of 96-hour paclitaxel in previously treated patients with advanced esophageal cancer. Cancer Invest. 2003;21:512–6.
Muro K, Hamaguchi T, Ohtsu A, et al. A phase II study of single-agent docetaxel in patients with metastatic esophageal cancer. Ann Oncol. 2004;15:955–9.
Kato K, Tahara M, Hironaka S, et al. A phase II study of paclitaxel by weekly 1-h infusion for advanced or recurrent esophageal cancer in patients who had previously received platinum-based chemotherapy. Cancer Chemother Pharmacol. 2011;67:1265–72.
Shim HJ, Cho SH, Hwang JE, et al. Phase II study of docetaxel and cisplatin chemotherapy in 5-fluorouracil/cisplatin pretreated esophageal cancer. Am J Clin Oncol. 2010;33:624–8.
Ford HER, Marshall A, Bridgewater JA, et al. Docetaxel versus active symptom control for refractory oesophagogastric adenocarcinoma (COUGAR-02): an open-label, phase 3 randomised controlled trial. Lancet Oncol. 2014;15:78–86.
Matsuoka K, Nakagawa F, Kobunai T, et al. Trifluridine/tipiracil overcomes the resistance of human gastric 5-fluorouracil-refractory cells with high thymidylate synthase expression. Oncotarget. 2018;9:13438–50.
Corsello SM, Nagari RT, Spangler RD, et al. Discovering the anti-cancer potential of non-oncology drugs by systematic viability profiling. Nat Cancer. 2020;1:235–48.
Mayer RJ, Van Cutsem E, Falcone A, et al. Randomized trial of TAS-102 for refractory metastatic colorectal cancer. N Engl J Med. 2015;372:1909–19.
Shitara K, Doi T, Dvorkin M, et al. Trifluridine/tipiracil versus placebo in patients with heavily pretreated metastatic gastric cancer (TAGS): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol. 2018;19:1437–48.
Nomura M, Iwasa S, Tsushima T, et al. Active salvage chemotherapy versus best supportive care for patients with recurrent or metastatic squamous cell carcinoma of the esophagus refractory or intolerable to fluorouracil, platinum, and taxane. Cancer Chemother Pharmacol. 2016;78:1209–16.
Eisenhauer EA, Therasse P, Bogaerts J, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer. 2009;45:228–47.
Japan Esophageal Society. Japanese classification of esophageal cancer, 11th edition: part I. Esophagus. 2017;14:1–36.
Japan Esophageal Society. Japanese classification of esophageal cancer, 11th edition: part II and III. Esophagus. 2017;14:37–65.
Kudo T, Hamamoto Y, Kato K, et al. Nivolumab treatment for oesophageal squamous-cell carcinoma: an open-label, multicentre, phase 2 trial. Lancet Oncol. 2017;18:631–9.
Kato K, Cho BC, Takahashi M, et al. Nivolumab versus chemotherapy in patients with advanced oesophageal squamous cell carcinoma refractory or intolerant to previous chemotherapy (ATTRACTION-3): a multicentre, randomised, open-label, phase 3 trial. Lancet Oncol. 2019;20:1506–17.
Kojima T, Shah MA, Muro K, et al. Randomized phase III KEYNOTE-181 study of pembrolizumab versus chemotherapy in advanced esophageal cancer. J Clin Oncol. 2020;38:4138–48.
Ohashi S, Kikuchi O, Nakai Y, et al. Synthetic lethality with trifluridine/tipiracil and checkpoint kinase 1 inhibitor for esophageal squamous cell carcinoma. Mol Cancer Ther. 2020;19:1363–72.
Acknowledgements
We would like to especially thank the patients who participated in this study and their families. We would also like to thank all investigators and research coordinators who participated in this study at the seven participating sites.
Funding
This study was sponsored by Taiho Pharmaceuticals Co., Ltd. (Joint Research: Project number 150150700012).
Author information
Authors and Affiliations
Contributions
YM, OK, TH, KM, HK, and MM contributed to the study design and conception. HH, SH, TK, KK, TT, and RI were involved in the data acquisition. RU and AK were trial statisticians. HT was responsible for the quality control of the data and algorithms. All authors contributed substantially to the analysis and interpretation of the data. All authors were involved in the drafting and revision of the manuscript, and all approved the final, submitted version of the manuscript.
Corresponding author
Ethics declarations
Ethical statement
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Conflict of interest
Yukiko Mori received honoraria from Daiichi Sankyo and Nipponkayaku. Osamu Kikuchi declares that he has no conflict of interest. Takahiro Horimatsu declares that he has no conflict of interest. Hiroki Hara received grants from Astellas, AstraZeneca, Bayer, BeiGene, Boehringer Ingelheim, Chugai, Daiichi Sankyo, Dainippon Sumitomo, Eisai, Elevar Therapeutics, GSK, Incyte, Merck Biopharma, MSD, Ono, Pfizer, and Taiho, consulting fees from Boehringer Ingelheim, Daiichi Sankyo, Dainippon Sumitomo, Lilly, MSD, Ono, honoraria from Bayer, Bristol-Myers Squibb, Chugai, Daiichi Sankyo, Kyowa Hakko Kirin, Lilly, Merck Biopharma, MSD, Ono, Sanofi, Taiho, Takeda, and Yakult. Shuichi Hironaka received research funding and honoraria from Taiho. Takashi Kojima received grants from MSD, Ono, Bristol-Myers Squibb, Astellas Amgen BioPharma, Taiho Pharmaceutical, Chugai, and Shionogi and received honoraria from Ono, Bristol-Myers Squibb, MSD, Astellas Pharma, Merk, and Oncolys BioPharma. Ken Kato received research funding from Ono, participation on a Data Safety Monitoring Board or Advisory Board for Bristol-Myers Squibb, MSD, and Roche, and was a leadership or fiduciary role on other boards, society, join committees, or advocacy groups for BMS, MSD, and BeiGene. Takahiro Tsushima declares that he has no conflict of interest. Ryu Ishihara declares that he has no conflict of interest. Kumi Mukai declares that she has no conflict of interest. Ryuji Uozumi received consulting fees from Eisai, Sawai Pharmaceutical, and CAC croit. Harue Tada declares that she has no conflict of interest. Hiroi Kasai declares that she has no conflict of interest. Atsushi Kawaguchi declares that he has no conflict of interest. Manabu Muto received funding support from Taiho.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Mori, Y., Kikuchi, O., Horimatsu, T. et al. Multicenter phase II study of trifluridine/tipiracil for esophageal squamous carcinoma refractory/intolerant to 5-fluorouracil, platinum compounds, and taxanes: the ECTAS study. Esophagus 19, 444–451 (2022). https://doi.org/10.1007/s10388-021-00905-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10388-021-00905-2