Abstract
Purpose
To evaluate the efficacy and safety of weekly paclitaxel (Taxol®) in patients with advanced or recurrent esophageal cancer.
Methods
Fifty-three patients with recurrent or advanced esophageal cancer who had previously received platinum-based chemotherapy were treated with paclitaxel 100 mg/m2 once weekly by 1-h infusion on days 1, 8, 15, 22, 29, and 36 of a 49-day cycle. Fifty-two patients were evaluable for efficacy and 53 for safety. Forty-one (77%) patients had recurrent, and 12 (23%) had advanced disease. Most patients (52/53) had squamous cell carcinoma, and one had adenocarcinoma.
Results
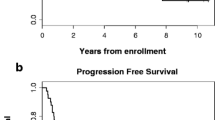
A median of 2 cycles was delivered (range 1–8). The overall response rate was 44.2% (23/52; 95% confidence interval (CI) 30.5, 58.7%), with 4 patients (7.7%) achieving complete response. The median duration of response was 4.8 months, and median overall survival was 10.4 months. The most common Grade 3 or 4 adverse events were neutropenia (52.8%), leukopenia (45.3%), anorexia (9.4%), and fatigue (9.4%). Adverse events resulted in treatment discontinuation in 34.0% of patients and dose reductions in 43.4%. There were no treatment-related deaths.
Conclusions
Weekly paclitaxel demonstrated efficacy and manageable toxicity in patients with advanced or recurrent esophageal cancer and may be a treatment option for this population.


Similar content being viewed by others
References
Jemal A, Siegel R, Ward E, Murray T, Xu J, Thun MJ (2007) Cancer statistics, 2007. CA Cancer J Clin 57:43–66
Parkin DM, Bray F, Ferlay J, Pisani P (2005) Global cancer statistics, 2002. CA Cancer J Clin 55:74–108
Blot WJ, McLaughlin JK (1999) The changing epidemiology of esophageal cancer. Semin Oncol 26:2–8
Devesa SS, Blot WJ, Fraumeni JF Jr (1998) Changing patterns in the incidence of esophageal and gastric carcinoma in the United States. Cancer 83:2049–2053
Vizcaino AP, Moreno V, Lambert R, Parkin DM (2002) Time trends incidence of both major histologic types of esophageal carcinomas in selected countries, 1973–1995. Int J Cancer 99:860–868
Akiyama H, Tsurumaru M, Udagawa H, Kajiyama Y (1994) Radical lymph node dissection for cancer of the thoracic esophagus. Ann Surg 220:364–372
Baba M, Aikou T, Yoshinaka H, Natsugoe S, Fukumoto T, Shimazu H, Akazawa K (1994) Long-term results of subtotal esophagectomy with three-field lymphadenectomy for carcinoma of the thoracic esophagus. Ann Surg 219:310–316
Fujita H, Kakegawa T, Yamana H, Shima I, Toh Y, Tomita Y, Fujii T, Yamasaki K, Higaki K, Noake T (1995) Mortality and morbidity rates, postoperative course, quality of life, and prognosis after extended radical lymphadenectomy for esophageal cancer. Comparison of three-field lymphadenectomy with two-field lymphadenectomy. Ann Surg 222:654–662
Mera K, Otsu A (2003) Practice of chemotherapy in esophageal cancer. Clin Gastroenterol 6:291–297
Iizuka T, Kakegawa T, Ide H, Ando N, Watanabe H, Tanaka O, Takagi I, Isono K, Ishida K, Arimori M (1992) Phase II evaluation of cisplatin and 5-fluorouracil in advanced squamous cell carcinoma of the esophagus: a Japanese Esophageal Oncology Group Trial. Jpn J Clin Oncol 22:172–176
Ajani JA, Ilson DH, Daugherty K, Pazdur R, Lynch PM, Kelsen DP (1994) Activity of taxol in patients with squamous cell carcinoma and adenocarcinoma of the esophagus. J Natl Cancer Inst 86:1086–1091
Gong Y, Ren L, Zhou L, Zhu J, Huang M, Zhou X, Wang J, Lu Y, Hou M, Wei Y (2009) Phase II evaluation of nedaplatin and paclitaxel in patients with metastatic esophageal carcinomas. Cancer Chemother Pharmacol 64:327–333
El Rayes BF, Shields A, Zalupski M, Heilbrun LK, Jain V, Terry D, Ferris A, Philip PA (2004) A phase II study of carboplatin and paclitaxel in esophageal cancer. Ann Oncol 15:960–965
Ilson DH, Ajani J, Bhalla K, Forastiere A, Huang Y, Patel P, Martin L, Donegan J, Pazdur R, Reed C, Kelsen DP (1998) Phase II trial of paclitaxel, fluorouracil, and cisplatin in patients with advanced carcinoma of the esophagus. J Clin Oncol 16:1826–1834
Lin CC, Hsu CH, Cheng JC, Wang HP, Lee JM, Yeh KH, Yang CH, Lin JT, Cheng AL, Lee YC (2007) Concurrent chemo radiotherapy with twice weekly paclitaxel and cisplatin followed by esophagectomy for locally advanced esophageal cancer. Ann Oncol 18:93–98
Abu-Rustum NR, Aghajanian C, Barakat RR, Fennelly D, Shapiro F, Spriggs D (1997) Salvage weekly paclitaxel in recurrent ovarian cancer. Semin Oncol 24:S15
Fennelly D, Aghajanian C, Shapiro F, O’Flaherty C, McKenzie M, O’Connor C, Tong W, Norton L, Spriggs D (1997) Phase I and pharmacologic study of paclitaxel administered weekly in patients with relapsed ovarian cancer. J Clin Oncol 15:187–192
Horiguchi J, Rai Y, Tamura K, Taki T, Hisamatsu K, Ito Y, Seriu T, Tajima T (2009) Phase II study of weekly paclitaxel for advanced or metastatic breast cancer in Japan. Anticancer Res 29:625–630
DeVore RF III, Jagasia M, Johnson DH (1997) Paclitaxel by either 1 h or 24 h infusion in combination with carboplatin in advanced non-small cell lung cancer: preliminary results comparing sequential phase II trials. Semin Oncol 24:S12
Maier-Lenz H, Hauns B, Haering B, Koetting J, Mross K, Unger C, Bauknecht T, du BA, Meerpohl HG, Hollaender N, Diergarten K (1997) Phase I study of paclitaxel administered as a 1 h infusion: toxicity and pharmacokinetics. Semin Oncol 24:S19
Ilson DH, Wadleigh RG, Leichman LP, Kelsen DP (2007) Paclitaxel given by a weekly 1 h infusion in advanced esophageal cancer. Ann Oncol 18:898–902
Nokihara H, Tamura T, Matsumoto Y (2002) Weekly paclitaxel in solid tumor, a phase I trial. Jpn J Lung Cancer 42. Abstract E-13
Seidman A, Hudis C, Albanell J, Tong W, Tepler I, Currie V, Moynahan M, Theodoulou M, Gollub M, Baselga J, Norton L (1998) Dose-dense therapy with weekly 1 h paclitaxel infusions in the treatment of metastatic breast cancer. J Clin Oncol 16:3353–3361
Therasse P, Arbuck SG, Eisenhauer EA, Wanders J, Kaplan RS, Rubinstein L, Verweij J, Van Glabbeke M, van Oosterom AT, Christian MC, Gwyther SG (2000) New guidelines to evaluate the response to treatment in solid tumors. European organization for research and treatment of cancer, national cancer institute of the United States, national cancer institute of Canada. J Natl Cancer Inst 92:205–216
Conroy T, Etienne PL, Adenis A, Wagener DJ, Paillot B, Francois E, Bedenne L, Jacob JH, Seitz JF, Bleiberg H, Van Pottelsberghe C, Van Glabbeke M, Delgado FM, Merle S, Wils J (1996) Phase II trial of vinorelbine in metastatic squamous cell esophageal carcinoma. European organization for research and treatment of cancer gastrointestinal treat cancer cooperative group. J Clin Oncol 14:164–170
Muro K, Hamaguchi T, Ohtsu A, Boku N, Chin K, Hyodo I, Fujita H, Takiyama W, Ohtsu T (2004) A phase II study of single-agent docetaxel in patients with metastatic esophageal cancer. Ann Oncol 15:955–959
Cho SH, Chung IJ, Song SY, Yang DH, Byun JR, Kim YK, Lee JJ, Na KJ, Kim HJ (2005) Bi-weekly chemotherapy of paclitaxel and cisplatin in patients with metastatic or recurrent esophageal cancer. J Korean Med Sci 20:618–623
Takashima A, Shiraro K, Hirashima Y, Takahari D, Okita N, Akatsuka S, Eguchi Nakajima T, Matsubara J, Yasui H, Asakawa T, Kato K, Hamguchi T, Muro K, Yamada Y, Shimada Y (2008) Chemosensitivity of patients with recurrent esophageal cancer receiving perioperative chemotherapy. Dis Esophagus 21:607–611
Acknowledgments
The Paclitaxel Esophageal Cancer Study Group comprises the following institutions: National Cancer Center Hospital, Tokyo; National Cancer Center Hospital East, Chiba; Shizuoka Cancer Center, Shizuoka; Aichi Cancer Center Central Hospital, Aichi; Osaka Medical College, Osaka; Tochigi Cancer Center, Tochigi; Kinki University School of Medicine, Osaka; The Cancer Institute Hospital of JFCR, Tokyo; Kitasato University East Hospital, Kanagawa; Osaka Medical Center for Cancer and Cardiovascular Diseases, Osaka; Saitama Cancer Center, Saitama; and Graduate School of Medicine, Osaka University, Osaka, Japan. This study was supported by a grant from Bristol-Myers K.K.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kato, K., Tahara, M., Hironaka, S. et al. A phase II study of paclitaxel by weekly 1-h infusion for advanced or recurrent esophageal cancer in patients who had previously received platinum-based chemotherapy. Cancer Chemother Pharmacol 67, 1265–1272 (2011). https://doi.org/10.1007/s00280-010-1422-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00280-010-1422-x