Abstract
Background
Neoadjuvant chemotherapy (NAC) followed by esophagectomy can improve the prognosis of locally advanced esophageal cancer (LAEC). However, LAEC reportedly recurred in 17–21% of patients within 6 months post surgery. Thus, current treatment strategies may be inadequate for LAECs with poor prognosis. Preoperative identification of patients with poor prognosis might aid in modification of treatment strategies. This study aimed to evaluate the usefulness of the maximum standardized uptake value change rate (ΔSUVmax) in predicting treatment effects on the primary lesion, prognosis, and LAEC recurrence.
Methods
This study involved 220 esophageal cancer patients who underwent esophagectomy after NAC at three facilities in Japan. The optimal cut-off point for ΔSUVmax in predicting tumor regression grade (TRG) was calculated and used to assess the correlation between ΔSUVmax and postoperative survival.
Results
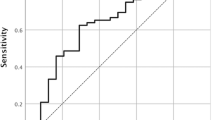
The optimal cut-off point for ΔSUVmax was 0.5. The 5-year overall survival rate in patients with ΔSUVmax ≥ 0.5 was significantly higher than that in patients with ΔSUVmax < 0.5 (71.5% vs. 50.5%, P = 0.001). Multivariate analysis identified ΔSUVmax (hazards ratio, 0.496; P = 0.004) as an independent prognostic factor. Among 199 patients evaluated for recurrence, 24 (12.1%) showed recurrence within 6 months post surgery. Univariate analysis revealed ΔSUVmax as the only predictor for early recurrence (odds ratio, 0.222; P = 0.004).
Conclusion
ΔSUVmax before and after NAC is clinically useful as it could help predict TRG, survival outcome, and early recurrence within 6 months post esophagectomy and is easily obtainable in general clinical practice. We believe that it may also help determine suitable treatment strategies for LAEC.



Similar content being viewed by others
References
van Hagen P, Hulshof MC, van Lanschot JJ, et al. Preoperative chemoradiotherapy for esophageal or junctional cancer. N Engl J Med. 2012;366:2074–84.
Ando N, Kato H, Igaki H, et al. A randomized trial comparing postoperative adjuvant chemotherapy with cisplatin and 5-fluorouracil versus preoperative chemotherapy for localized advanced squamous cell carcinoma of the thoracic esophagus (JCOG9907). Ann Surg Oncol. 2012;19:68–74.
Takahashi K, Miyashita M, Nomura T, et al. Serum p53 antibody as a predictor of early recurrence in patients with postoperative esophageal squamous cell carcinoma. Dis Esophagus. 2007;20:117–22.
Yoshida N, Baba Y, Shigaki H, et al. Risk factors of early recurrence within 6 months after esophagectomy following neoadjuvant chemotherapy for resectable advanced esophageal squamous cell carcinoma. Int J Clin Oncol. 2016;21:1071–8.
Hamai Y, Emi M, Ibuki Y, et al. Early recurrence and cancer death after trimodal therapy for esophageal squamous cell carcinoma. Anticancer Res. 2019;39:1433–40.
Oguma J, Ozawa S, Koyanagi K, et al. Prognostic significance of pathological tumor response and residual nodal metastasis in patients with esophageal squamous cell carcinoma after neoadjuvant chemotherapy followed by surgery. Esophagus. 2019;16:395–401.
Sugimura K, Miyata H, Shinno N, et al. Prognostic factors for esophageal squamous cell carcinoma treated with neoadjuvant docetaxel/cisplatin/5-fluorouracil followed by surgery. Oncology. 2019;97:348–55.
Izumi D, Yoshida N, Watanabe M, et al. Tumor/normal esophagus ratio in (18)F-fluorodeoxyglucose positron emission tomography/computed tomography for response and prognosis stratification after neoadjuvant chemotherapy for esophageal squamous cell carcinoma. J Gastroenterol. 2016;51:788–95.
Kaida H, Kitajima K, Nakajo M, et al. Predicting tumor response and prognosis to neoadjuvant chemotherapy in esophageal squamous cell carcinoma patients using PERCIST: a multicenter study in Japan. Eur J Nucl Med Mol Imaging. 2021;48:3666–82.
Makino T, Yamasaki M, Tanaka K, et al. Metabolic tumor volume change predicts long-term survival and histological response to preoperative chemotherapy in locally advanced esophageal cancer. Ann Surg. 2019;270:1090–5.
Sonoda A, Yoshida N, Shiraishi S, et al. Total lesion glycolysis ratio in positron emission tomography/computed tomography images during neoadjuvant chemotherapy can predict pathological tumor regression grade and prognosis in patients with locally advanced squamous cell carcinoma of the esophagus. Ann Surg Oncol. 2021;28:167–74.
Sobin LH, Gospodarowicz MK, Wittekind C, et al. International Union against Cancer. TNM classification of malignant tumours. 7th ed. Chichester: Wiley-Blackwell; 2010.
Iwasaki Y, Terashima M, Mizusawa J, et al. Gastrectomy with or without neoadjuvant S-1 plus cisplatin for type 4 or large type 3 gastric cancer (JCOG0501): an open-label, phase 3, randomized controlled trial. Gastric Cancer. 2021;24:492–502.
Iwatsuki M, Orita H, Kobayashi K, et al. Phase II study of S-1 and oxaliplatin as neoadjuvant chemotherapy for locally advanced adenocarcinoma of the gastric or esophagogastric junction: KSCC1601. Gastric Cancer. 2021. https://doi.org/10.1007/s10120-021-01218-0.
Noda K, Nishiwaki Y, Kawahara M, et al. Irinotecan plus cisplatin compared with etoposide plus cisplatin for extensive small-cell lung cancer. N Engl J Med. 2002;346:85–91.
Hara H, Tahara M, Daiko H, et al. Phase II feasibility study of preoperative chemotherapy with docetaxel, cisplatin, and fluorouracil for esophageal squamous cell carcinoma. Cancer Sci. 2013;104:1455–60.
Koizumi W, Kim YH, Fujii M, et al. Addition of docetaxel to S-1 without platinum prolongs survival of patients with advanced gastric cancer: a randomized study (START). J Cancer Res Clin Oncol. 2014;140:319–28.
Kuwano H, Nishimura Y, Oyama T, et al. Guidelines for diagnosis and treatment of carcinoma of the esophagus April 2012 edited by the Japan Esophageal Society. Esophagus. 2015;12:1–30.
Japan Esophageal Society. Japanese Classification of Esophageal Cancer, 11th Edition: part I. Esophagus. 2017;14:1–36.
Japan Esophageal Society. Japanese Classification of Esophageal Cancer, 11th Edition: part II and III. Esophagus. 2017;14:37–65.
Miyata H, Yamasaki M, Takahashi T, et al. Determinants of response to neoadjuvant chemotherapy for esophageal cancer using 18F-fluorodeoxiglucose positron emission tomography (18F-FDG-PET). Ann Surg Oncol. 2014;21:575–82.
Smithers BM, Couper GC, Thomas JM, et al. Positron emission tomography and pathological evidence of response to neoadjuvant therapy in adenocarcinoma of the esophagus. Dis Esophagus. 2008;21:151–8.
Gillies RS, Middleton MR, Han C, et al. Role of positron emission tomography-computed tomography in predicting survival after neoadjuvant chemotherapy and surgery for oesophageal adenocarcinoma. Br J Surg. 2012;99:239–45.
Tani Y, Nakajima M, Kikuchi M, et al. 18F-fluorodeoxyglucose positron emission tomography for evaluating the response to neoadjuvant chemotherapy in advanced esophageal cancer. Anticancer Res. 2016;36:367–73.
Ohsawa M, Hamai Y, Emi M, et al. Tumor response in esophageal squamous cell carcinoma treated with neoadjuvant chemotherapy followed by surgery. Anticancer Res. 2020;40:1153–60.
Taniyama Y, Sakurai T, Hikage M, et al. How does presurgical chemotherapy influence the efficiency of treatment for esophageal cancer recurrence after curative esophagectomy? Thorac Cancer. 2019;10:769–74.
Yamamoto S, Kato K, Daiko H, et al. Feasibility study of nivolumab as neoadjuvant chemotherapy for locally esophageal carcinoma: FRONTiER (JCOG1804E). Future Oncol. 2020;16:1351–7.
Al-Batran SE, Homann N, Pauligk C, et al. Perioperative chemotherapy with fluorouracil plus leucovorin, oxaliplatin, and docetaxel versus fluorouracil or capecitabine plus cisplatin and epirubicin for locally advanced, resectable gastric or gastro-oesophageal junction adenocarcinoma (FLOT4): a randomised, phase 2/3 trial. Lancet. 2019;393:1948–57.
Zhu ZJ, Hu Y, Zhao YF, et al. Early recurrence and death after esophagectomy in patients with esophageal squamous cell carcinoma. Ann Thorac Surg. 2011;91:1502–8.
Kosugi S, Kanda T, Yajima K, et al. Risk factors that influence early death due to cancer recurrence after extended radical esophagectomy with three-field lymph node dissection. Ann Surg Oncol. 2011;18:2961–7.
Nakajima M, Muroi H, Kikuchi M, et al. Adverse prognostic factors of advanced esophageal cancer in patients undergoing induction therapy with docetaxel, cisplatin and 5-fluorouracil. Anticancer Res. 2018;38:911–8.
Acknowledgements
This study was conducted to update the Japanese Classification of Esophageal Cancer by the Japan Esophageal Society. The authors sincerely thank Yuichiro Doki, MD, and Koji Tanaka, MD, for their valuable assistance and direction in conducting this study.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Ethical statement
This study was conducted in accordance with the principles of the Declaration of Helsinki. The Institutional Review Board at each institute approved the present study protocol (registry number: 19374 [Chiba University], 2011 [Kumamoto University], 2020-1-114 [Tohoku University]). The requirement for obtaining written informed consent from patients was waived because of the retrospective design of the study.
Conflict of interest
Dr. Naoya Yoshida is affiliated with a department supported by Chugai Pharmaceutical Co., Ltd., but declares no conflicts of interest in relation to this research. The rest of the authors have no financial conflicts of interest related to this study.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Murakami, K., Yoshida, N., Taniyama, Y. et al. Maximum standardized uptake value change rate before and after neoadjuvant chemotherapy can predict early recurrence in patients with locally advanced esophageal cancer: a multi-institutional cohort study of 220 patients in Japan. Esophagus 19, 205–213 (2022). https://doi.org/10.1007/s10388-021-00896-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10388-021-00896-0