Abstract
Background
Positron emission tomography (PET) response criteria in solid tumors were recently proposed as a standardized method for the metabolic and quantitative assessment of response to chemotherapy. However, use of these criteria is limited in many institutions because of the need for exclusive software. This study was designed to clarify whether tumor to normal esophageal (T/N) ratio on 18F-fluorodeoxyglucose PET/computed tomography could predict response to neoadjuvant chemotherapy and stratify prognosis in patients with esophageal squamous cell carcinoma (ESCC).
Methods
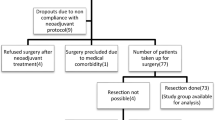
Clinicopathological data were collected for 73 patients with ESCC who received neoadjuvant chemotherapy with docetaxel, cisplatin, and 5-fluorouracil followed by curative resection. The right liver lobe and normal esophagus were utilized as reference tissues for diagnosing complete metabolic response (CMR). Statistical methods included Kaplan–Meier analysis and univariate and multivariate Cox proportional hazards regression analyses.
Results
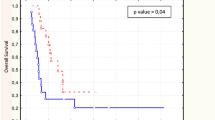
CMR was achieved in 24 patients on the basis of maximum standardized uptake value (SUVmax) and in 11 on the basis of SUVmax evaluation with T/N ratio. Although prognosis was poorer in patients who achieved CMR than partial metabolic response based on SUVmax, the responses were significantly correlated with disease-free survival (DFS) based on SUVmax evaluation with T/N ratio (P = 0.0011). Receiver operating characteristic curve analysis showed that SUVmax evaluation with T/N ratio was the best predictor of pGrade 3. Multivariate analysis showed that SUVmax evaluation with T/N ratio was an independent predictor of DFS in patients with pGrade 1 pathologic response.
Conclusions
SUVmax evaluation with T/N ratio is useful for evaluating the effects of neoadjuvant chemotherapy in patients with ESCC.




Similar content being viewed by others
References
Burmeister BH, Smithers BM, Gebski V, et al. Surgery alone versus chemoradiotherapy followed by surgery for resectable cancer of the oesophagus: a randomised controlled phase III trial. Lancet Oncol. 2005;6:659–68.
Bedenne L, Michel P, Bouche O, et al. Chemoradiation followed by surgery compared with chemoradiation alone in squamous cancer of the esophagus: FFCD 9102. J Clin Oncol. 2007;25:1160–8.
Sjoquist KM, Burmeister BH, Smithers BM, et al. Survival after neoadjuvant chemotherapy or chemoradiotherapy for resectable oesophageal carcinoma: an updated meta-analysis. Lancet Oncol. 2011;12:681–92.
van Hagen P, Hulshof MC, van Lanschot JJ, et al. Preoperative chemoradiotherapy for esophageal or junctional cancer. N Engl J Med. 2012;366:2074–84.
Ando N, Kato H, Igaki H, et al. A randomized trial comparing postoperative adjuvant chemotherapy with cisplatin and 5-fluorouracil versus preoperative chemotherapy for localized advanced squamous cell carcinoma of the thoracic esophagus (JCOG9907). Ann Surg Oncol. 2012;19:68–74.
Hoffman PC, Haraf DJ, Ferguson MK, et al. Induction chemotherapy, surgery, and concomitant chemoradiotherapy for carcinoma of the esophagus: a long-term analysis. Ann Oncol. 1998;9:647–51.
Watanabe M, Nagai Y, Kinoshita K, et al. Induction chemotherapy with docetaxel/cisplatin/5-fluorouracil for patients with node-positive esophageal cancer. Digestion. 2011;83:146–52.
Watanabe M, Baba Y, Yoshida N, et al. Outcomes of preoperative chemotherapy with docetaxel, cisplatin, and 5-fluorouracil followed by esophagectomy in patients with resectable node-positive esophageal cancer. Ann Surg Oncol. 2014;21:2838–44.
Yoshida N, Watanabe M, Baba Y, et al. Influence of preoperative docetaxel, cisplatin, and 5-fluorouracil on the incidence of complications after esophagectomy for resectable advanced esophageal cancer. Dis Esophagus. 2014;27:374–9.
Eisenhauer EA, Therasse P, Bogaerts J, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer. 2009;45:228–47.
Miyata H, Yoshioka A, Yamasaki M, et al. Tumor budding in tumor invasive front predicts prognosis and survival of patients with esophageal squamous cell carcinomas receiving neoadjuvant chemotherapy. Cancer. 2009;115:3324–34.
Wahl RL, Jacene H, Kasamon Y, Lodge MA. From RECIST to PERCIST: evolving considerations for PET response criteria in solid tumors. J Nucl Med. 2009;50(Suppl 1):122S–50S.
Society JE. Japanese classification of esophageal cancer. 10th ed. Tokyo: Kanehara; 2008.
Mima K, Okabe H, Ishimoto T, et al. CD44s regulates the TGF-beta-mediated mesenchymal phenotype and is associated with poor prognosis in patients with hepatocellular carcinoma. Cancer Res. 2012;72:3414–23.
Lordick F, Ott K, Krause BJ, et al. PET to assess early metabolic response and to guide treatment of adenocarcinoma of the oesophagogastric junction: the MUNICON phase II trial. Lancet Oncol. 2007;8:797–805.
Fletcher JW, Djulbegovic B, Soares HP, et al. Recommendations on the use of 18F-FDG PET in oncology. J Nucl Med. 2008;49:480–508.
Van den Abbeele AD. The lessons of GIST–PET and PET/CT: a new paradigm for imaging. Oncologist. 2008;13(Suppl 2):8–13.
Milano A, Perri F, Ciarmiello A, Caponigro F. Targeted-therapy and imaging response: a new paradigm for clinical evaluation? Rev Recent Clin Trials. 2011;6:259–65.
Schroder O, Trojan J, Zeuzem S, Baum RP. Limited value of fluorine-18-fluorodeoxyglucose PET for the differential diagnosis of focal liver lesions in patients with chronic hepatitis C virus infection. Nuklearmedizin. 1998;37:279–85.
Keramida G, Potts J, Bush J, et al. Hepatic steatosis is associated with increased hepatic FDG uptake. Eur J Radiol. 2014;83:751–5.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflicts of interest.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Izumi, D., Yoshida, N., Watanabe, M. et al. Tumor/normal esophagus ratio in 18F-fluorodeoxyglucose positron emission tomography/computed tomography for response and prognosis stratification after neoadjuvant chemotherapy for esophageal squamous cell carcinoma. J Gastroenterol 51, 788–795 (2016). https://doi.org/10.1007/s00535-015-1150-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00535-015-1150-4