Abstract
Purpose
To evaluate the association between central serous chorioretinopathy (CSC) susceptibility genes and choroidal parameters in a large Japanese cohort.
Study design
Retrospective cohort study.
Methods
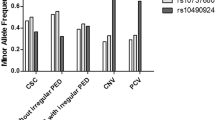
Of the 9850 individuals in the Nagahama study whose second visit was between 2013 and 2016, those with optical coherence tomography (OCT) images with enhanced depth imaging (EDI), axial length, and genome-wide single nucleotide polymorphism (SNP) genotyping data were included. We calculated subfoveal choroidal thickness (SFCT), choroidal vascularity index (CVI), normalized choroidal intensity (NCI), and vertical asymmetry of choroidal thickness. Genome-wide quantitative trait locus (QTL) analyses were performed for each parameter. We screened for four CSC susceptibility SNPs: CFH rs800292, TNFRSF10A rs13278062, GATA5 rs6061548, and VIPR2 rs3793217. Whenever an SNP was not included in the genotyping data after quality control, its proxy SNP was selected.
Results
In total, 4586 participants were evaluated. CFH rs800292 was significantly associated with SFCT (P < 0.001) and CVI (P < 0.001). VIPR2 rs3793217 was significantly associated with SFCT (P < 0.001) but not with CVI. Whereas, TNFRSF10A rs13254617 and GATA5 rs6061548 were not significantly associated with SFCT or CVI. None of these SNPs was associated with NCIEDI and asymmetry of choroidal thickness.
Conclusion
CFH, VIPR2, TNFRSF10A, and GATA5 showed different association patterns with choroidal parameters. Although the mechanism of CSC pathogenesis by choroidal changes is not fully understood, this finding suggests that each gene may be involved in different mechanisms of CSC development. Our genetic study provides a basis for understanding the role of CSC susceptibility genes.
Similar content being viewed by others
References
Miyake M, Tsujikawa A, Yamashiro K, Ooto S, Oishi A, Tamura H, et al. Choroidal neovascularization in eyes with choroidal vascular hyperpermeability. Invest Ophthalmol Vis Sci. 2014;55:3223–30.
Gemenetzi M, De Salvo G, Lotery AJ. Central serous chorioretinopathy: an update on pathogenesis and treatment. Eye. 2010;24:1743–56.
Kitzmann AS, Pulido JS, Diehl NN, Hodge DO, Burke JP. The incidence of central serous chorioretinopathy in Olmsted County, Minnesota, 1980–2002. Ophthalmology. 2008;115:169–73.
Kido A, Miyake M, Tamura H, Hiragi S, Kimura T, Ohtera S, et al. Incidence of central serous chorioretinopathy (2011-2018): a nationwide population-based cohort study of Japan. Br J Ophthalmol. 2021;bjophthalmol-2021-319403.
Klein ML, Van Buskirk EM, Friedman E, Gragoudas E, Chandra S. Experience with nontreatment of central serous choroidopathy. Arch Ophthalmol. 1974;91:247–50.
Nicholson B, Noble J, Forooghian F, Meyerle C. Central serous chorioretinopathy: update on pathophysiology and treatment. Surv Ophthalmol. 2013;58:103–26.
Bujarborua D. Long-term follow-up of idiopathic central serous chorioretinopathy without laser. Acta Ophthalmol Scand. 2001;79:417–21.
Bouzas EA, Karadimas P, Pournaras CJ. Central serous chorioretinopathy and glucocorticoids. Surv Ophthalmol. 2002;47:431–48.
Mrejen S, Balaratnasingam C, Kaden TR, Bottini A, Dansingani K, Bhavsar KV, et al. Long-term visual outcomes and causes of vision loss in chronic central serous chorioretinopathy. Ophthalmology. 2019;126:576–88.
Aisu N, Miyake M, Hosoda Y, Mori Y, Takahashi A, Muraoka Y, et al. Effectiveness of reduced-fluence photodynamic therapy for chronic central serous chorioretinopathy. Ophthalmology Science. 2022;2: 100152.
Pang CE, Freund KB. Pachychoroid neovasculopathy. Retina. 2015;35:1–9.
Warrow DJ, Hoang QV, Freund KB. Pachychoroid pigment epitheliopathy. Retina. 2013;33:1659–72.
Miyake M, Ooto S, Yamashiro K, Takahashi A, Yoshikawa M, Akagi-Kurashige Y, et al. Pachychoroid neovasculopathy and age-related macular degeneration. Sci Rep. 2015;5:16204.
Yanagi Y. Pachychoroid disease: a new perspective on exudative maculopathy. Jpn J Ophthalmol. 2020;64:323–37.
Yamashiro K, Hosoda Y, Miyake M, Ooto S, Tsujikawa A. Characteristics of pachychoroid diseases and age-related macular degeneration: multimodal imaging and genetic backgrounds. J Clin Med. 2020;9:2034.
Hosoda Y, Miyake M, Yamashiro K, Ooto S, Takahashi A, Oishi A, et al. Deep phenotype unsupervised machine learning revealed the significance of pachychoroid features in etiology and visual prognosis of age-related macular degeneration. Sci Rep. 2020;10:18423.
Cheung CMG, Lee WK, Koizumi H, Dansingani K, Lai TYY, Freund KB. Pachychoroid Dis EYE. 2019;33:14–33.
Yamashiro K, Hosoda Y, Miyake M, Takahashi A, Ooto S, Tsujikawa A. Hypothetical pathogenesis of age-related macular degeneration and pachychoroid diseases derived from their genetic characteristics. Jpn J Ophthalmol. 2020;64:555–67.
Mori Y, Miyake M, Hosoda Y, Miki A, Takahashi A, Muraoka Y, et al. Genome-wide survival analysis for macular neovascularization development in central serous chorioretinopathy revealed shared genetic susceptibility with polypoidal choroidal vasculopathy. Ophthalmology. 2022;129:1034–42.
Miki A, Kondo N, Yanagisawa S, Bessho H, Honda S, Negi A. Common variants in the complement factor H gene confer genetic susceptibility to central serous chorioretinopathy. Ophthalmology. 2014;121:1067–72.
Hosoda Y, Yoshikawa M, Miyake M, Tabara Y, Ahn J, Woo SJ, et al. CFH and VIPR2 as susceptibility loci in choroidal thickness and pachychoroid disease central serous chorioretinopathy. Proc Natl Acad Sci USA. 2018;115:6261–6.
Hosoda Y, Miyake M, Schellevis RL, Boon CJF, Hoyng CB, Miki A, et al. Genome-wide association analyses identify two susceptibility loci for pachychoroid disease central serous chorioretinopathy. Commun Biol. 2019;2:468.
Guyer DR, Yannuzzi LA, Slakter JS, Sorenson JA, Ho A, Orlock D. Digital indocyanine green videoangiography of central serous chorioretinopathy. Arch Ophthalmol. 1994;112:1057–62.
Yannuzzi LA. Central serous chorioretinopathy: a personal perspective. Am. J. Ophthalmol. 2010. p. 361–3.
Spaide RF, Koizumi H, Pozzoni MC. Enhanced depth imaging spectral-domain optical coherence tomography. Am J Ophthalmol. 2008;146:496–500.
Agrawal R, Salman M, Tan K-A, Karampelas M, Sim DA, Keane PA, et al. Choroidal vascularity index (CVI)–A novel optical coherence tomography parameter for monitoring patients with panuveitis? PLoS ONE. 2016;11: e0146344.
Agrawal R, Gupta P, Tan K-A, Cheung CMG, Wong T-Y, Cheng C-Y. Choroidal vascularity index as a measure of vascular status of the choroid: Measurements in healthy eyes from a population-based study. Sci Rep. 2016;6:21090.
Balasubramanian S, Lei J, Nittala MG, Velaga SB, Haines J, Pericak-Vance MA, et al. Association of drusen volume with choroidal parameters in nonneovascular age-related macular degeneration. Retina. 2017;37:1880–7.
Velaga SB, Nittala MG, Vupparaboina KK, Jana S, Chhablani J, Haines J, et al. Choroidal vascularity index and choroidal thickness in eyes with reticular pseudodrusen. Retina. 2020;40:612–7.
Imamura Y, Fujiwara T, Margolis R, Spaide RF. Enhanced depth imaging optical coherence tomography of the choroid in central serous chorioretinopathy. Retina. 2009;29:1469–73.
Baek J, Lee JH, Jung BJ, Kook L, Lee WK. Morphologic features of large choroidal vessel layer: age-related macular degeneration, polypoidal choroidal vasculopathy, and central serous chorioretinopathy. Graefes Arch Clin Exp Ophthalmol. 2018;256:2309–17.
Kuroda S, Ikuno Y, Yasuno Y, Nakai K, Usui S, Sawa M, et al. Choroidal thickness in central serous chorioretinopathy. Retina. 2013;33:302–8.
Brandl C, Helbig H, Gamulescu MA. Choroidal thickness measurements during central serous chorioretinopathy treatment. Int Ophthalmol. 2014;34:7–13.
Agrawal R, Chhablani J, Tan K-A, Shah S, Sarvaiya C, Banker A. Choroidal vascularity index in central serous chorioretinopathy. Retina. 2016;36:1646–51.
Chhablani J, Rasheed M, Goud A, Mohamed A, Vupparaboina K. Change in choroidal vascularity in acute central serous chorioretinopathy. Indian J Ophthalmol. 2018;66:530.
Kim R-Y, Chung DH, Kim M, Park Y-H. Use of choroidal vascularity index for choroidal structural evaluation in central serous chorioretinopathy with choroidal neovascularization. Retina. 2020;40:1395–402.
Yang J, Wang E, Yuan M, Chen Y. Three-dimensional choroidal vascularity index in acute central serous chorioretinopathy using swept-source optical coherence tomography. Graefes Arch Clin Exp Ophthalmol. 2020;258:241–7.
Mori Y, Miyake M, Hosoda Y, Uji A, Nakano E, Takahashi A, et al. Distribution of choroidal thickness and choroidal vessel dilation in healthy Japanese individuals. Ophthalmol Sci. 2021;1: 100033.
Miyake M, Yamashiro K, Tabara Y, Suda K, Morooka S, Nakanishi H, et al. Identification of myopia-associated WNT7B polymorphisms provides insights into the mechanism underlying the development of myopia. Nat Commun. 2015;6:6689.
Nakata I, Yamashiro K, Nakanishi H, Akagi-Kurashige Y, Miyake M, Tsujikawa A, et al. Prevalence and characteristics of age-related macular degeneration in the Japanese population: the Nagahama study. Am J Ophthalmol. 2013;156:1002-9.e2.
K. Setoh and F. Matsuda. (2022). Cohort Profile: The Nagahama Prospective Genome Cohort for Comprehensive Human Bioscience (The Nagahama Study). In: Yano, M., Matsuda, F., Sakuntabhai, A., Hirota, S. (eds) Socio-Life Science and the COVID-19 Outbreak. Economics, Law, and Institutions in Asia Pacific. Springer, Singapore.
Hiroe T, Kishi S. Dilatation of asymmetric vortex vein in central serous chorioretinopathy. Ophthalmol Retina. 2018;2:152–61.
Yamada H, Hangai M, Nakano N, Takayama K, Kimura Y, Miyake M, et al. Asymmetry analysis of macular inner retinal layers for glaucoma diagnosis. Am J Ophthalmol. 2014;158:1318-29.e3.
Delaneau O, Marchini J, 1000 Genomes Project Consortium, 1000 Genomes Project Consortium. Integrating sequence and array data to create an improved 1000 Genomes Project haplotype reference panel. Nat Commun. 2014;5:3934.
Das S, Forer L, Schönherr S, Sidore C, Locke AE, Kwong A, et al. Next-generation genotype imputation service and methods. Nat Genet. 2016;48:1284–7.
Devlin B, Roeder K. Genomic control for association studies. Biometrics. 1999;55:997–1004.
Mori K, Ishikawa K, Fukuda Y, Ji R, Wada I, Kubo Y, et al. TNFRSF10A downregulation induces retinal pigment epithelium degeneration during the pathogenesis of age-related macular degeneration and central serous chorioretinopathy. Hum Mol Genet. 2022;31:2194–206.
Acknowledgements
We are grateful to the Nagahama City Office and the nonprofit organization, Zeroji Club for their help in conducting the Nagahama Study. We would also like to thank Ms. Hatsue Hamanaka for her assistance with genotyping.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of interest
K. Morino, None; M. Miyake, Grants or Contracts (Novartis), Payment or honoraria for lectures, presentations, speakers bureaus, manuscript writing or educational events (Bayer, Kowa, Alcon, HOYA, Novartis, AMO, Santen, Senju, Johnson & Johnson); T. Kamei, None; T. Kawaguchi, None; Y. Mori, Payment or honoraria for lectures, presentations, speakers bureaus, manuscript writing or educational events (Santen); Y. Hosoda, None; A. Uji, Payment or honoraria for lectures, presentations, speakers bureaus, manuscript writing or educational events (Canon, Bayer, Novartis, Santen, Senju, HOYA); K. Yamashiro, Payment or honoraria for lectures, presentations, speakers bureaus, manuscript writing or educational events (Novartis, Bayer, Santen, Alcon, Senju, Kowa, Canon, Chugai); F. Matsuda, None; A. Tsujikawa, Grants or Contracts (Canon, Findex, Santen, Kowa, Pfizer, Senju, Wakamoto, Alcon, Novartis, Otsuka, Bayer, Nitten), Consulting fees (Senju, Bayer, Novartis, HOYA, Ellex, MSD, Allegan, Eisai, Daiich-Sankyo, Chugai, KYOWA HAKKO BIO), Payment or honoraria for lectures, presentations, speakers bureaus, manuscript writing or educational events (Bayer, Senju, Novartis, Santen, Alcon, AbbVie, AMO, Kowa, Canon, Otsuka, Wakamoto, NIDEK).
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Corresponding Author: Masahiro Miyake
Supplementary Information
Below is the link to the electronic supplementary material.
About this article
Cite this article
Morino, K., Miyake, M., Kamei, T. et al. Association between central serous chorioretinopathy susceptibility genes and choroidal parameters. Jpn J Ophthalmol 66, 504–510 (2022). https://doi.org/10.1007/s10384-022-00945-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10384-022-00945-w